523968
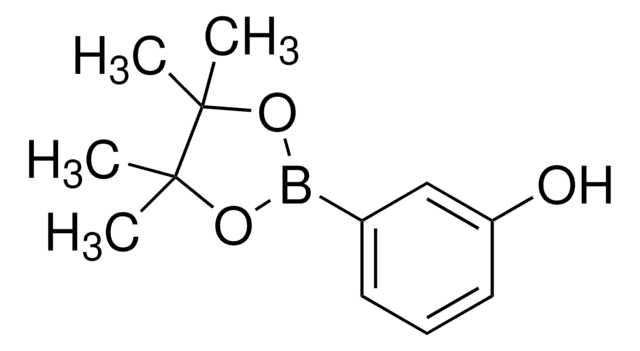
3-Hydroxyphenylboronic acid
≥95.0%
Synonym(s):
3-Hydroxybenzeneboronic acid, m-Hydroxybenzeneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
HOC6H4B(OH)2
CAS Number:
Molecular Weight:
137.93
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
mp
210-213 °C (dec.) (lit.)
SMILES string
OB(O)c1cccc(O)c1
InChI
1S/C6H7BO3/c8-6-3-1-2-5(4-6)7(9)10/h1-4,8-10H
InChI key
WFWQWTPAPNEOFE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3-Hydroxyphenylboronic acid (3-HPBA) can be used as a reagent:
- In Suzuki-Miyaura coupling reactions with aryl halides for the formation of C-C bond in the presence of Pd catalyst.
- To synthesize boron/nitrogen-doped polymer nano/microspheres by hydrothermal polymerization with formaldehyde and ammonia.
- To prepare carbon quantum dots based on 3-HPBA as selective fructose sensor.
- In the development of modified electrodes for electrochemical biosensors.
Footnote
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Diana M A Crista et al.
Journal of fluorescence, 29(1), 265-270 (2019-01-07)
The selective fluorescence sensing of fructose was achieved by fluorescence quenching of the emission of hydrothermal-synthesized carbon quantum dots prepared by 3-hydroxyphenylboronic acid. Quantification of fructose was possible in aqueous solutions with pH of 9 (Limit of Detection LOD and
Facile synthesis of monodisperse bulk boron-and nitrogen-doped carbon nano/microspheres
Zhao J, et al.
Journal of Material Chemistry A, 6(46), 23780-23786 (2018)
Synthesis of hyperbranched polythiophene with a controlled degree of branching via catalyst-transfer Suzuki-Miyaura coupling reaction
Segawa Y, et al.
Polym. Chem., 4(4), 1208-1215 (2013)
Recent progress in electrochemical biosensors based on phenylboronic acid and derivatives
Anzai J-i
Materials Science and Engineering, C, 67, 737-746 (2016)
Mingyan Zhu et al.
ACS combinatorial science, 14(2), 124-134 (2011-12-21)
As a continuation of our previous report (J. Comb. Chem.2010, 12, 548-558), we accomplished the diversity-oriented synthesis of polyheterocyclic small-molecule library with privileged benzopyran substructure. To ensure the synthetic efficiency, we utilized the solid-phase parallel platform and the fluorous-tag-based solution-phase
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service