496758
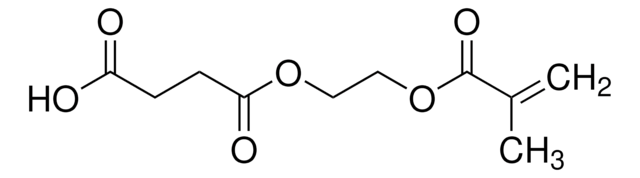
Bis[2-(methacryloyloxy)ethyl] phosphate
Synonym(s):
2-Hydroxyethyl methacrylate phosphate, Bis(2-methacryloyloxyethyl) acid phosphate, Bis(2-methacryloyloxyethyl) hydrogen phosphate, Bis(methacryloyloxyethyl) hydrogen phosphate, Bis(methacryloyloxyethyl) phosphate, Bis[2-(methacryloyloxy)ethyl] phosphate, Di(2-methacryloyloxyethyl) phosphate, Di-2-methacryloyloxyethyl acid phosphate
About This Item
Recommended Products
refractive index
n20/D 1.47 (lit.)
Quality Level
bp
221 °C (lit.)
density
1.28 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(=C)C(=O)OCCOP(O)(=O)OCCOC(=O)C(C)=C
InChI
1S/C12H19O8P/c1-9(2)11(13)17-5-7-19-21(15,16)20-8-6-18-12(14)10(3)4/h1,3,5-8H2,2,4H3,(H,15,16)
InChI key
NXBXJOWBDCQIHF-UHFFFAOYSA-N
Related Categories
General description
Application
- As a bifunctional crosslinker to synthesize superabsorbent polymers (SAPs) via free radical polymerization. These SAPs can be used for agricultural applications.
- To prepare self-etching primer formulations for enamel and dentine. Primers with BMEP exhibit good wetting, etching, and penetration in both dentine and enamel.
- As a precursor to synthesize fire retardant nanocomposites via suspension polymerization.
Features and Benefits
- Presence of dimethacrylate groups enables cross-linking and improves copolymerization.
- Hydrophilicity and short carbon chains can enhance wetting.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![[2-(Methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide 95%](/deepweb/assets/sigmaaldrich/product/structures/217/219/73c91e1c-0ee4-4b3d-bead-a6dc3d09d1da/640/73c91e1c-0ee4-4b3d-bead-a6dc3d09d1da.png)