B32807
Benzyltriphenylphosphonium chloride
99%
Sinónimos:
BTPPC, NSC 116712, Triphenyl(phenylmethyl)phosphonium chloride
About This Item
Productos recomendados
Quality Level
assay
99%
form
powder
reaction suitability
reaction type: C-C Bond Formation
mp
≥300 °C (lit.)
SMILES string
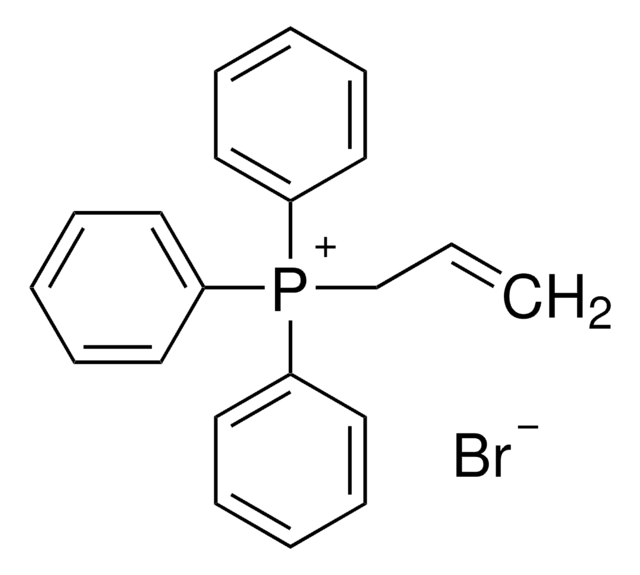
[Cl-].C(c1ccccc1)[P+](c2ccccc2)(c3ccccc3)c4ccccc4
InChI
1S/C25H22P.ClH/c1-5-13-22(14-6-1)21-26(23-15-7-2-8-16-23,24-17-9-3-10-18-24)25-19-11-4-12-20-25;/h1-20H,21H2;1H/q+1;/p-1
InChI key
USFRYJRPHFMVBZ-UHFFFAOYSA-M
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- Platinum chloro-tetrazole complexes via azidation.
- Trans-stilbenes and cinnamates via Wittig olefination.
- Achiral N-hydroxyformamide inhibitors of ADAM-TS4 and ADAM-TS5 for osteoarthritis treatment.
- Pentiptycenes for use as light-driven molecular brakes.
- Archipelago structures for formation of petroleum asphaltenes.
It is also used as a crosslinking agent for tube-like natural halloysite / fluorelastomer nanocomposites.
Reactant for synthesis of:
- Platinum chloro tetrazole complexes via azidation
- Trans-stilbenes and cinnamates via Wittig olefination
- Achiral N-hydroxyformamide inhibitors of ADAM-TS4 and ADAM-TS5 for osteoarthritis treatment
- Pentiptycenes for use as light-driven molecular brakes
Reactant for formation of archipelago structures for formation of petroleum asphaltenes
signalword
Danger
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - STOT RE 1 - STOT SE 3
target_organs
Lungs,nasal cavity, Respiratory system
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
572.0 °F - closed cup
flash_point_c
300 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico