911666
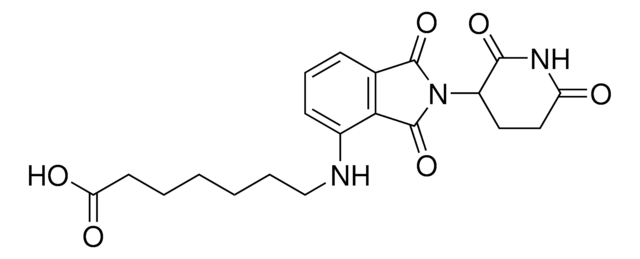
Pomalidomide-C6-NH2 hydrochloride
≥95%
Sinónimos:
4-((6-Aminohexyl)amino)-2-(2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione hydrochloride, Crosslinker−E3 ligase ligand conjugate, Pomalidomide conjugate, Protein degrader building block for PROTAC® research, Template for synthesis of targeted protein degrader
About This Item
Productos recomendados
ligand
pomalidomide
assay
≥95%
form
powder
reaction suitability
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
functional group
amine
storage temp.
2-8°C
SMILES string
O=C(C(CC1)N(C2=O)C(C3=C2C=CC=C3NCCCCCCN)=O)NC1=O.Cl
Application
Other Notes
Portal: Building PROTAC® Degraders for Targeted Protein Degradation
Proteolysis Targeting Chimeras for the Selective Degradation of Mcl-1/Bcl-2 Derived from Nonselective Target Binding Ligands
Chemoselective Synthesis of Lenalidomide-Based PROTAC Library Using Alkylation Reaction
Identification of New Small-Molecule Inducers of Estrogen-related Receptor α (ERRα) Degradation
Discovery of MD-224 as a First-in-Class, Highly Potent and Efficacious PROTAC MDM2 Degrader Capable of Achieving Complete and Durable Tumor Regression
Legal Information
related product
signalword
Danger
hcodes
pcodes
Hazard Classifications
Repr. 1B
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico