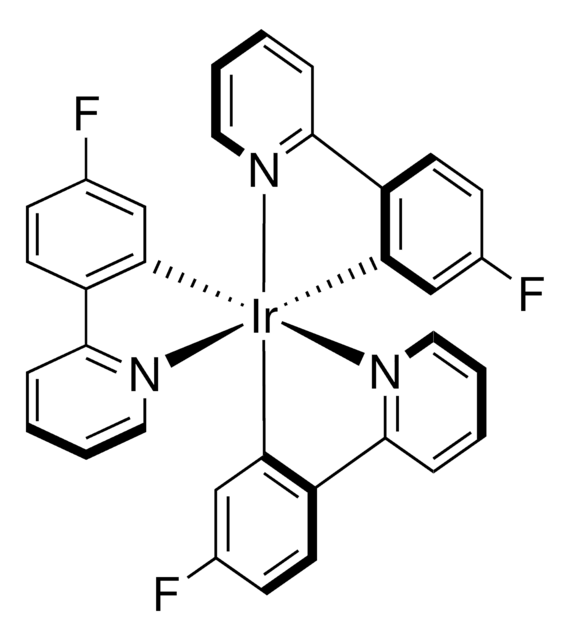
901409
[Ir(dF(Me)ppy)2(dtbbpy)]PF6
Sinónimos:
Iridium(III) bis[2-(2,4-difluorophenyl)-5-methylpyridine-N,C20]-4,40-di-tert-butyl-2,20-bipyridine hexafluorophosphate
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C42H40F10IrN4P
Número de CAS:
Peso molecular:
1013.97
UNSPSC Code:
12161600
NACRES:
NA.22
Productos recomendados
form
solid
Quality Level
reaction suitability
core: iridium
reaction type: Photocatalysis
reagent type: catalyst
photocatalyst activation
450 nm
Categorías relacionadas
Application
[Ir(dF(Me)ppy)2(dtbbpy)]PF6 is a photocatalyst readily facilitates hydroamination of olefins as well as the decarboxylative arylation and vinylation of carboxylic acids via metallaphotoredox catalysis.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Other Notes
Catalytic intermolecular hydroaminations of unactivated olefins with secondary alkyl amines
Merging photoredox and nickel catalysis: Decarboxylative cross-coupling of carboxylic acids with vinyl halides
Enhanced luminescent iridium(III) complexes bearing aryltriazole cyclometallated ligands
Site-Selective and Stereoselective C-H Alkylations of Carbohydrates via Combined Diarylborinic Acid and Photoredox Catalysis
Merging photoredox and nickel catalysis: Decarboxylative cross-coupling of carboxylic acids with vinyl halides
Enhanced luminescent iridium(III) complexes bearing aryltriazole cyclometallated ligands
Site-Selective and Stereoselective C-H Alkylations of Carbohydrates via Combined Diarylborinic Acid and Photoredox Catalysis
related product
Referencia del producto
Descripción
Precios
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lot/Batch Number
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Victoria Dimakos et al.
Journal of the American Chemical Society, 141(13), 5149-5153 (2019-03-23)
Diphenylborinic acid serves as a cocatalyst for site- and stereoselective C-H alkylation reactions of carbohydrates under photoredox conditions using quinuclidine as the hydrogen atom transfer mediator. Products arising from selective abstraction of the equatorial hydrogens of cis-1,2-diol moieties, followed by
Adam Noble et al.
Journal of the American Chemical Society, 137(2), 624-627 (2014-12-19)
Decarboxylative cross-coupling of alkyl carboxylic acids with vinyl halides has been accomplished through the synergistic merger of photoredox and nickel catalysis. This new methodology has been successfully applied to a variety of α-oxy and α-amino acids, as well as simple
Sébastien Ladouceur et al.
Inorganic chemistry, 50(22), 11514-11526 (2011-10-04)
Herein we report the synthesis of 4-aryl-1-benzyl-1H-1,2,3-triazoles (atl), made via "Click chemistry" and their incorporation as cyclometallating ligands into new heteroleptic iridium(III) complexes containing diimine (N(^)N) ancillary ligands 2,2'-bipyridine (bpy) and 4,4'-di-tert-butyl-2,2'-bipyridine (dtBubpy). Depending on decoration, these complexes emit from
Andrew J Musacchio et al.
Science (New York, N.Y.), 355(6326), 727-730 (2017-02-18)
The intermolecular hydroamination of unactivated alkenes with simple dialkyl amines remains an unsolved problem in organic synthesis. We report a catalytic protocol for efficient additions of cyclic and acyclic secondary alkyl amines to a wide range of alkyl olefins with
Strongly Blue Luminescent Cationic Iridium(III) Complexes with an Electron-Rich Ancillary Ligand: Evaluation of Their Optoelectronic and Electrochemiluminescence Properties.
Ladouceur S, et al.
European Journal of Inorganic Chemistry, 2013(30), 5329-5343 (2013)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico![(Ir[dF(CF3)ppy]2(dtbpy))PF6](/deepweb/assets/sigmaaldrich/product/structures/982/913/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09/640/02dd8ddd-6deb-40a0-ab9b-07b18f1abb09.png)
![[Ir{dFCF3ppy}2(bpy)]PF6](/deepweb/assets/sigmaaldrich/product/structures/180/924/79119ac4-7d62-429d-b23d-a14c012c6050/640/79119ac4-7d62-429d-b23d-a14c012c6050.png)
![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)
![[Ir(dFCF3ppy)2-(5,5’-dCF3bpy)]PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/422/901/e00f3148-fb86-4f94-9e79-21d064c3f327/640/e00f3148-fb86-4f94-9e79-21d064c3f327.png)
![Ir[dFFppy]2-(4,4′-dCF3bpy)PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/816/772/b116c17c-e6b2-4c95-be64-45a5a851d823/640/b116c17c-e6b2-4c95-be64-45a5a851d823.png)

![[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride](/deepweb/assets/sigmaaldrich/product/structures/471/091/6faa29b1-bf8a-4d87-90b2-4cc55e082620/640/6faa29b1-bf8a-4d87-90b2-4cc55e082620.png)


![[Ir(p-F(Me)ppy)2-(4,4′-dtbbpy)]PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/231/079/a5445824-9d4b-4c84-9c5f-f3acbcc75fd4/640/a5445824-9d4b-4c84-9c5f-f3acbcc75fd4.png)
![Ir[dF(t-Bu)-ppy]3](/deepweb/assets/sigmaaldrich/product/structures/254/294/d0fb19e5-05b2-4c1b-990b-a99fa60b3e73/640/d0fb19e5-05b2-4c1b-990b-a99fa60b3e73.png)
![Tris[2-phenylpyridinato-C2,N]iridium(III) 97%](/deepweb/assets/sigmaaldrich/product/structures/167/234/658d0b76-d31d-4fd5-8041-e04e207227c9/640/658d0b76-d31d-4fd5-8041-e04e207227c9.png)