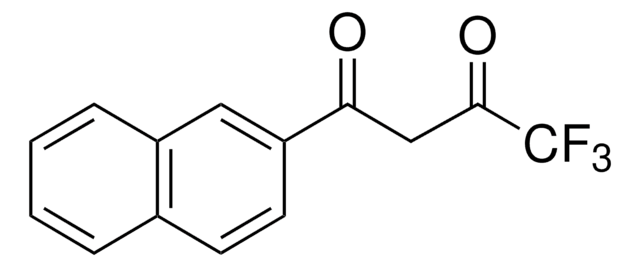
426016
4,4,4-Trifluoro-1-(2-furyl)-1,3-butanedione
99%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C8H5F3O3
Número de CAS:
Peso molecular:
206.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
99%
form
liquid
refractive index
n20/D 1.528 (lit.)
bp
203 °C (lit.)
mp
19-21 °C (lit.)
density
1.391 g/mL at 25 °C (lit.)
functional group
fluoro
ketone
storage temp.
2-8°C
SMILES string
FC(F)(F)C(=O)CC(=O)c1ccco1
InChI
1S/C8H5F3O3/c9-8(10,11)7(13)4-5(12)6-2-1-3-14-6/h1-3H,4H2
InChI key
OWLPCALGCHDBCN-UHFFFAOYSA-N
General description
4,4,4-Trifluoro-1-(2-furyl)-1,3-butanedione (furoyltrifluoroacetone, FTFA) is a β-diketone. Its cytotoxic activity against human cultured tumor and normal cells has been evaluated. Reports suggest that 4,4,4-trifluoro-1-(2-furyl)-1,3-butanedione partially inhibits the oxidation of ferrocyanide in ETP (electron transport particles) isolated from beef heart mitochondria. Its reaction with N,N,N′,N′-tetramethylalkyl diamines to form ionic adducts has been investigated. The conformational analysis of the enol and keto form of FTFA has been reported.
Application
4,4,4-Trifluoro-1-(2-furyl)-1,3-butanedione (tfa) may be used in the following studies:
- As capping ligand in the synthesis of [Eu(tfa)3]2bpm complexes (bpm=2,2′-bipyrimidine).
- As reagent in the multistep synthesis of [13CD2]benzylamine.
- As reagent in the synthesis of 3-trifluoromethyl-2-arylcarbonylquinoxaline 1,4-di-N-oxide derivatives by reacting with corresponding benzofurazan oxides.
- In the efficient syntheses of perfluoroalkyl substituted azoles.
- Synthesis of 2-arylcarbonyl-3-trifluoromethylquinoxaline 1,4-di-N-oxide derivatives.
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
188.6 °F - closed cup
flash_point_c
87 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Acyclic tertiary diamines and 1, 4, 7,10-tetraazacyclododecane with fluorine-containing β-diketones: Leading to low melting ionic adducts.
Gupta OD, et al.
Journal of Fluorine Chemistry, 126(8), 1222-1229 (2005)
Adoración Marin et al.
Experimental parasitology, 118(1), 25-31 (2007-07-07)
Derivatives of 3-trifluoromethyl-2-arylcarbonylquinoxaline 1,4-di-N-oxide (4b-g, 5b-g, 6a-g) were synthesized and evaluated for their capacity to inhibit the growth of chloroquine-resistant Plasmodium falciparum FCB1 strain in culture. Compound 7-chloro-2-(2-furylcarbonyl)-3-trifluoromethyl-1,4-quinoxaline di-N-oxide (5g) was the most active being almost 5 times more active
Synthesis and biological evaluation of new 2-arylcarbonyl-3-trifluoromethylquinoxaline 1,4-di-N-oxide derivatives and their reduced analogues.
Solano B, et al.
Journal of Medicinal Chemistry, 50(22), 5485-5492 (2007)
Topography of the cristae membrane as elucidated by a new inhibitor, trifluorofurylbutanedione.
H J Harmon et al.
Biochemical and biophysical research communications, 55(1), 169-173 (1973-11-01)
Kensuke Nakano et al.
Anticancer research, 24(2B), 711-717 (2004-05-27)
A variety of beta-diketones were evaluated for their cytotoxic profiles against oral human normal and tumor cells. Among 22 compounds (BD1-22) tested, the cytotoxicity of 3-formylchromone (BD17) (CC50=7.8 microg/mL) against human oral squamous cell carcinoma (HSC-2) cells was higher than
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico