418218
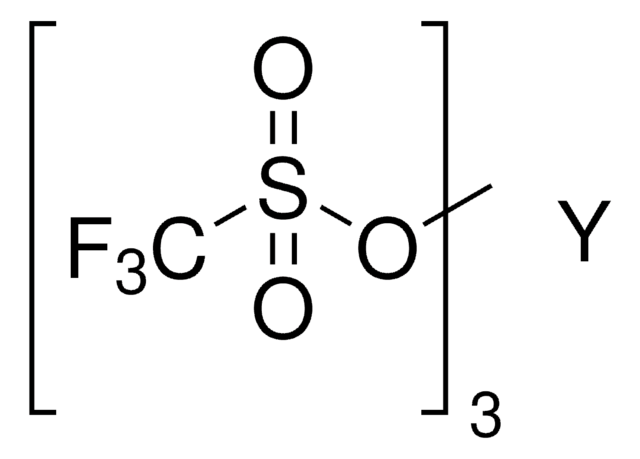
Scandium(III) triflate
99%
Sinónimos:
Sc(OTf)3, Scandium(III) trifluoromethanesulfonate, Trifluoromethanesulfonic acid scandium(III) salt
About This Item
Productos recomendados
Quality Level
assay
99%
form
powder
reaction suitability
core: scandium
reagent type: catalyst
SMILES string
[Sc+3].[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F.[O-]S(=O)(=O)C(F)(F)F
InChI
1S/3CHF3O3S.Sc/c3*2-1(3,4)8(5,6)7;/h3*(H,5,6,7);/q;;;+3/p-3
InChI key
HZXJVDYQRYYYOR-UHFFFAOYSA-K
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- Hydrothiolation reaction of aromatic and aliphatic thiols.
- Selective two-electron reduction of O2 by ferrocene derivatives.
- Vinylogous Friedel-Crafts alkylation of indoles and pyrroles in water.
- Synthesis of β-cyanoketones.
- Combination with triethylsilane to reductively open functionalized pyranoside rings.
- The key steps of synthesis of bullvalone via a stabilized sulfur ylide.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Friedel-Crafts acylation with Lewis acid catalysts forms monoacylated products via electrophilic aromatic substitution of arenes.
Friedel-Crafts acylation with Lewis acid catalysts forms monoacylated products via electrophilic aromatic substitution of arenes.
Friedel-Crafts acylation with Lewis acid catalysts forms monoacylated products via electrophilic aromatic substitution of arenes.
Friedel-Crafts acylation with Lewis acid catalysts forms monoacylated products via electrophilic aromatic substitution of arenes.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico