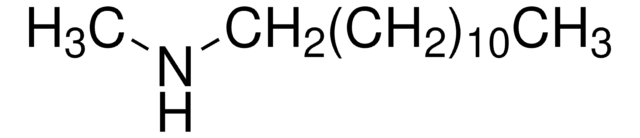36784
Didodecylamine
≥97.0% (GC)
Sinónimos:
Di-n-dodecylamine, Dilaurylamine, N,N-Didodecylamine, N-Dodecyl-1-dodecanamine
About This Item
Productos recomendados
assay
≥97.0% (GC)
form
powder
mp
45-49 °C
functional group
amine
SMILES string
CCCCCCCCCCCCNCCCCCCCCCCCC
InChI
1S/C24H51N/c1-3-5-7-9-11-13-15-17-19-21-23-25-24-22-20-18-16-14-12-10-8-6-4-2/h25H,3-24H2,1-2H3
InChI key
MJCJUDJQDGGKOX-UHFFFAOYSA-N
Categorías relacionadas
General description
Application
- to promote the crystal growth of 2D nanosheets of GdOCl with approximately square cross sections
- to investigate the effect of alkyl chain length of amine on phase transfer of aqueous synthesized gold nanoparticles (AuNPs) from water to toluene
- in the preparation of novel class of liquid crystalline complexes, by the interaction of copper nitrate
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico