328936
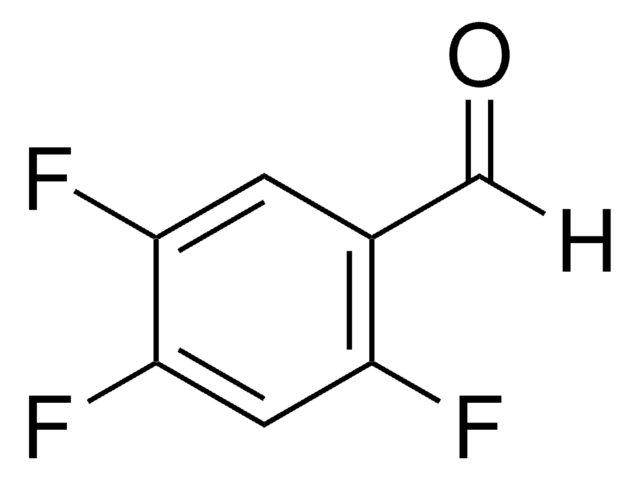
2,3,5,6-Tetrafluorobenzaldehyde
97%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
HC6F4CHO
Número de CAS:
Peso molecular:
178.08
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
97%
form
liquid
refractive index
n20/D 1.469 (lit.)
bp
178 °C (lit.)
density
1.525 g/mL at 25 °C (lit.)
functional group
aldehyde
fluoro
SMILES string
Fc1cc(F)c(F)c(C=O)c1F
InChI
1S/C7H2F4O/c8-4-1-5(9)7(11)3(2-12)6(4)10/h1-2H
InChI key
YIRYOMXPMOLQSO-UHFFFAOYSA-N
Categorías relacionadas
General description
2,3,5,6-Tetrafluorobenzaldehyde is a polysubstituted benzaldehyde and was evaluated as a substrate of PmHNL (Prunus mume hydroxynitrile lyase). Reaction of 2,3,5,6-tetrafluorobenzaldehyde with dipyrromethane was reported.
Application
2,3,5,6-Tetrafluorobenzaldehyde was used in the preparation of 1,3-bis(2,4,6-trimethylphenyl)-2-(2,3,5,6-tetrafluorophenyl)imidazolidine and 1,3-dimethyl-2-(2,3,5,6-tetrafluorophenyl)imidazolidine.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
165.2 °F - closed cup
flash_point_c
74 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Gregory W Nyce et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 10(16), 4073-4079 (2004-08-19)
The synthesis of N-heterocyclic carbene (NHC) adducts by condensation of diamines with appropriately substituted benzaldehydes is described. This simplified approach provides the NHC adduct without first having to generate the carbene followed by its protection. These adducts undergo thermal deprotection
Effects of aldehyde or dipyrromethane substituents on the reaction course leading to meso-substituted porphyrins.
Geier III, et al.
Tetrahedron, 60(50), 11435-11444 (2004)
A new (R)-hydroxynitrile lyase from< i> Prunus mume</i>: asymmetric synthesis of cyanohydrins.
Nanda S, et al.
Tetrahedron, 61(46), 10908-10916 (2005)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico