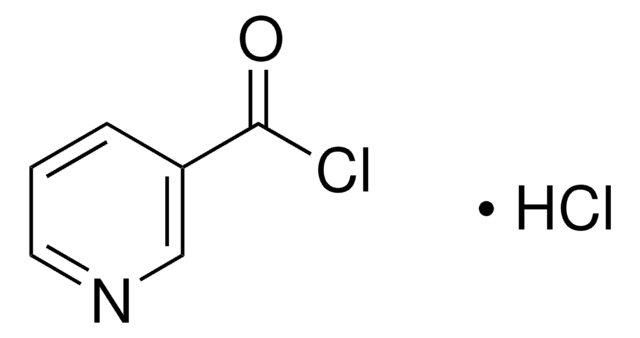
235253
(Diethylamino)sulfur trifluoride
95%
Sinónimos:
DAST, Diethylaminosulfur trifluoride
About This Item
Productos recomendados
Quality Level
assay
95%
form
liquid
bp
30-32 °C/3 mmHg (lit.)
density
1.22 g/mL at 25 °C (lit.)
functional group
amine
storage temp.
2-8°C
SMILES string
CCN(CC)S(F)(F)F
InChI
1S/C4H10F3NS/c1-3-8(4-2)9(5,6)7/h3-4H2,1-2H3
InChI key
CSJLBAMHHLJAAS-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- Fluorinating agent: reaction with alcohols and carbonyl compounds, Review
- Review on nucleophilic fluorination.
- Catalyst for Friedel-Crafts allylation using tertiary cyclopropyl silyl ethers and the rearrangement of homoallylic alcohols to unsaturated aldehydes.
- Early introduction of a fluoromethyl group stabilizes the epoxide during further manipulations in the synthesis of 26-fluoro-epothilone.
- Fluorinating agent for a variety of compounds, including thioethers, alkenols, and cyanohydrins.
- Reagent for gem difluorination of ketopipecolinic acids.
replaced by
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 3 - Self-react. D - Skin Corr. 1A
supp_hazards
Storage Class
5.2 - Organic peroxides and self-reacting hazardous materials
wgk_germany
WGK 3
flash_point_f
73.4 °F
flash_point_c
23 °C
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Diethylaminosulfur trifluoride (DAST) facilitates nucleophilic fluorination in selective reactions with alcohols, ketones, and other compounds.
Diethylaminosulfur trifluoride (DAST) facilitates nucleophilic fluorination in selective reactions with alcohols, ketones, and other compounds.
Diethylaminosulfur trifluoride (DAST) facilitates nucleophilic fluorination in selective reactions with alcohols, ketones, and other compounds.
Diethylaminosulfur trifluoride (DAST) facilitates nucleophilic fluorination in selective reactions with alcohols, ketones, and other compounds.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
![1-clorometil-4-fluoro-1,4-diazoniabiciclo[2,2.2]octano bis(tetrafluoroborato) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)