221015
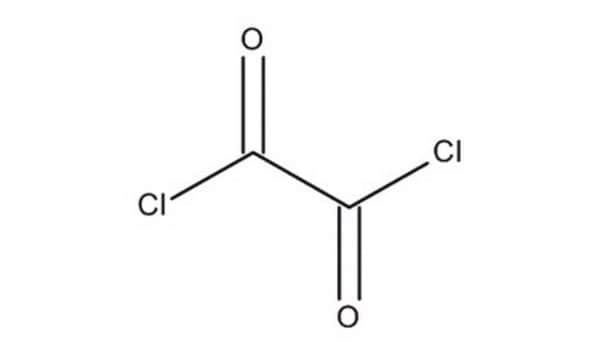
Oxalyl chloride
ReagentPlus®, ≥99%
Sinónimos:
Ethanedioyl dichloride
About This Item
Productos recomendados
vapor density
4.4 (vs air)
Quality Level
vapor pressure
150 mmHg ( 20 °C)
product line
ReagentPlus®
assay
≥99%
form
liquid
reaction suitability
reagent type: oxidant
impurities
<10 ppb Heavy metals
color
APHA: 0-150
refractive index
n20/D 1.429 (lit.)
bp
62-65 °C (lit.)
mp
−10-−8 °C (lit.)
density
1.5 g/mL at 20 °C (lit.)
functional group
acyl chloride
SMILES string
ClC(=O)C(Cl)=O
InChI
1S/C2Cl2O2/c3-1(5)2(4)6
InChI key
CTSLXHKWHWQRSH-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- Preparation of Mosher′s acid chloride by reacting with Mosher′s acid in the presence of DMF.
- Activation of dimethyl sulfoxide for use in the oxidation of long-chain alcohols to carbonyls.
- Activation of α-keto carboxylic acids and N-heterocyclic carboxylic acids for alkynylation to form ynediones and N-heterocyclic ynones, respectively.
- Synthesis of N-heterocyclic ynones and ynediones, used to activate carboxylic acids
- Chlorination and halogenation
- Three-component [3+2] cycloadditions
- Reactions with organostannanes
- Synthesis of cyclopentenones
- Carbonylations, used as a carbonyl synthon
Packaging
Legal Information
For use with
signalword
Danger
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - Water-react 1
supp_hazards
Storage Class
4.3 - Hazardous materials which set free flammable gases upon contact with water
wgk_germany
WGK 1
flash_point_f
51.8 °F - closed cup
flash_point_c
11.0 °C - closed cup
ppe
Faceshields, Gloves, Goggles
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico