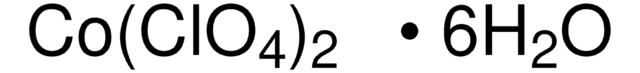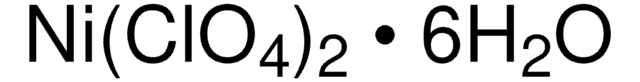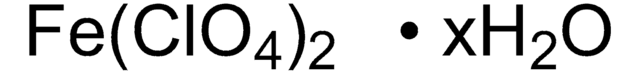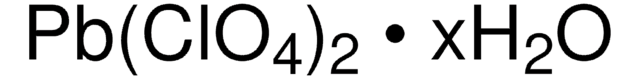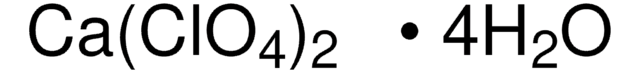215392
Copper(II) perchlorate hexahydrate
98%
Sinónimos:
Cupric perchlorate hexahydrate
About This Item
Productos recomendados
Quality Level
assay
98%
form
crystalline
reaction suitability
reagent type: oxidant
density
2.225 g/mL at 25 °C (lit.)
application(s)
battery manufacturing
SMILES string
O.O.O.O.O.O.[Cu++].[O-]Cl(=O)(=O)=O.[O-]Cl(=O)(=O)=O
InChI
1S/2ClHO4.Cu.6H2O/c2*2-1(3,4)5;;;;;;;/h2*(H,2,3,4,5);;6*1H2/q;;+2;;;;;;/p-2
InChI key
NHELIHXBJRANPL-UHFFFAOYSA-L
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- Methanol[1-(methoxymethanimidoyl)-2-(pyridin-2-ylmethyl)guanidine]bis(perchlorato)copper(II).: This study reports the synthesis and crystal structure of a new copper(II) complex with potential applications in materials science and catalysis. The research focuses on the structural characterization of the complex, which is crucial for understanding its reactivity and potential uses in various chemical processes (Meenongwa et al., 2012).
- Oxidative cleavage of DNA by a dipyridoquinoxaline copper(II) complex in the presence of ascorbic acid.: This research explores the catalytic activity of a copper(II) complex in oxidative DNA cleavage. The findings demonstrate the complex′s potential application in biochemical studies and the development of new catalytic processes for DNA manipulation and analysis (Santra et al., 2002).
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Ox. Sol. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
5.1A - Strongly oxidizing hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico