155071
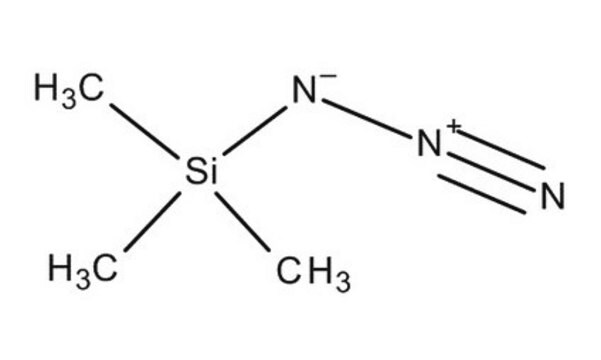
Azidotrimethylsilane
95%
Sinónimos:
Trimethylsilyl azide
About This Item
Productos recomendados
Quality Level
assay
95%
form
liquid
refractive index
n20/D 1.414 (lit.)
bp
52-53 °C/175 mmHg (lit.)
density
0.868 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
C[Si](C)(C)N=[N+]=[N-]
InChI
1S/C3H9N3Si/c1-7(2,3)6-5-4/h1-3H3
InChI key
SEDZOYHHAIAQIW-UHFFFAOYSA-N
General description
Application
- A nitrogen precursor to prepare GaN nanowire via metal-organic chemical vapor deposition method.
- An electrolyte additive in Li-O2 batteries. The addition of TMSN3 results in the formation of robust solid electrolyte interphase.
- An efficient reagent in the synthesis of tetrazoles, fullerenyl azide, and α-azido oximes.
- A silylating agent in the O-trimethyl silylation of alcohols and phenols.
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
42.8 °F - closed cup
flash_point_c
6 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico