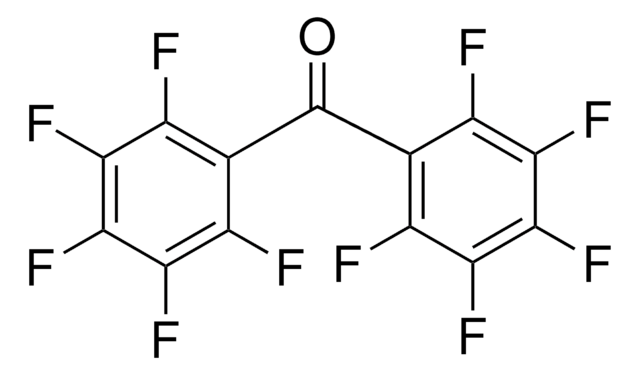
104353
Tetrafluoro-1,4-benzoquinone
97%
Sinónimos:
2,3,5,6-Tetrafluoroquinone, Fluoranil, Tetrafluorobenzoquinone
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
C6F4(=O)2
Número de CAS:
Peso molecular:
180.06
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
97%
form
solid
mp
183-186 °C (subl.) (lit.)
functional group
fluoro
ketone
SMILES string
FC1=C(F)C(=O)C(F)=C(F)C1=O
InChI
1S/C6F4O2/c7-1-2(8)6(12)4(10)3(9)5(1)11
InChI key
JKLYZOGJWVAIQS-UHFFFAOYSA-N
General description
Tetrafluoro-1,4-benzoquinone is a fluorinated building block, commonly used as a precursor for fluoro derivatives.
Application
Tetrafluoro-1,4-benzoquinone (fluoranil) can be used to prepare:
- Symmetrical or unsymmetrical ethers by coupling of two alcohols via the oxidation-reduction condensation reaction.
- Azocino[4,3-b]indole scaffold, which is used as an inetermediate to prepare (±)-dasycarpidone.
- Chiral and racemic charge-transfer (CT) complexes with binaphthol.
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Ben-Zhan Zhu et al.
Proceedings of the National Academy of Sciences of the United States of America, 104(45), 17575-17578 (2007-10-31)
We have shown previously that hydroxyl radicals (HO*) can be produced by H2O2 and halogenated quinones, independent of transition metal ions; however, the underlying molecular mechanism is still unclear. In the present study, using the electron spin resonance secondary radical
A convenient method for the preparation of symmetrical or unsymmetrical ethers by the coupling of two alcohols via a new type of oxidation-reduction condensation using tetrafluoro-1, 4-benzoquinone.
Shintou T and Mukaiyama T.
Chemistry Letters (Jpn), 11, 984-985 (2003)
Generation and spectroscopic characterization of the 2, 3, 5, 6-tetramethoxy-1, 4-benzosemiquinone reactive intermediate.
Mattar SM, et al.
Chemical Physics Letters, 352(1), 39-47 (2002)
Ken Okamoto et al.
Journal of the American Chemical Society, 125(41), 12416-12417 (2003-10-09)
Self-promoted electron transfer from a cobalt(II) porphyrin [Co(II)OEP] to p-fluoranil (F4Q) occurs, exhibiting a second-order dependence of the electron-transfer rate with respect to the F4Q concentration due to the formation of a strong complex between the dimer radical anion [(F4Q)2*-]
Complexation Behavior of Binaphthol/Tetrafluoro-1, 4-benzoquinone Charge-Transfer Complex.
Imai Y, et al.
Crystal Growth & Design, 9(5), 2393-2397 (2009)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico