102369
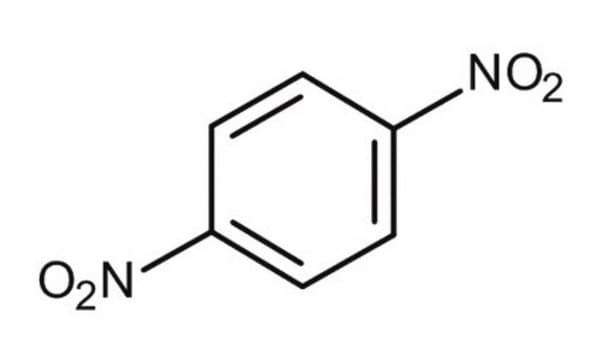
1,4-Dinitrobenzene
98%
Sinónimos:
p-dinitrobenzene, para-Dinitrobenzene
About This Item
Productos recomendados
Quality Level
assay
98%
bp
183.4 °C/34 mmHg (lit.)
mp
170-173 °C (lit.)
solubility
alcohol: soluble 1g in 300ml
boiling water: soluble 1g in 555ml
cold water: soluble 1g in 12,500ml
benzene: very slightly soluble
chloroform: very slightly soluble
ethyl acetate: very slightly soluble
density
1.625 g/mL at 25 °C (lit.)
functional group
nitro
SMILES string
[O-][N+](=O)c1ccc(cc1)[N+]([O-])=O
InChI
1S/C6H4N2O4/c9-7(10)5-1-2-6(4-3-5)8(11)12/h1-4H
InChI key
FYFDQJRXFWGIBS-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
Preparation Note
signalword
Danger
Hazard Classifications
Acute Tox. 1 Dermal - Acute Tox. 1 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 2
Storage Class
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
wgk_germany
WGK 3
flash_point_f
302.0 °F - closed cup
flash_point_c
150 °C - closed cup
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico