All Photos(1)
About This Item
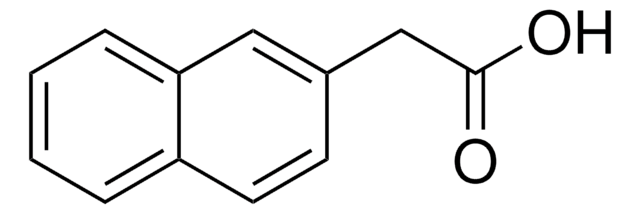
Linear Formula:
C10H7OCH2CO2H
CAS Number:
Molecular Weight:
202.21
Beilstein:
1074148
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
mp
151-154 °C (lit.)
SMILES string
OC(=O)COc1ccc2ccccc2c1
InChI
1S/C12H10O3/c13-12(14)8-15-11-6-5-9-3-1-2-4-10(9)7-11/h1-7H,8H2,(H,13,14)
InChI key
RZCJYMOBWVJQGV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
D Hössel et al.
Plant biology (Stuttgart, Germany), 7(1), 41-48 (2005-01-25)
A study of transport and action of synthetic auxin analogues can help to identify transporters and receptors of this plant hormone. Both aspects--transportability and action on growth--were tested with 2-naphthoxyacetic acid (2-NOA) and compared across several plant species. 2-NOA stimulates
Poonam Piplani et al.
Medicinal chemistry (Shariqah (United Arab Emirates)), 9(3), 371-378 (2012-08-28)
The present paper describes the design and synthesis of a series of some 2-naphthyloxy derivatives with their antiamnesic activity using mice as the animal model and piracetam as the reference drug. All the synthesized compounds were characterized by spectroscopic techniques
Asuman Karadeniz et al.
Toxicology and industrial health, 27(9), 840-848 (2011-04-23)
In this study, the mutagenic and recombinogenic effects of indole-3-acetic acid (IAA), a plant growth regulator naturally synthesized in plants but produced synthetically, and β-naphthoxyacetic acid (BNOA), a synthetic plant growth regulator widely used in agricultural regions, were investigated using
M Illangasekare et al.
Science (New York, N.Y.), 267(5198), 643-647 (1995-02-03)
An RNA has been selected that rapidly aminoacylates its 2'(3') terminus when provided with phenylalanyl-adenosine monophosphate. That is, the RNA accelerates the same aminoacyl group transfer catalyzed by protein aminoacyl-transfer RNA synthetases. The best characterized RNA reaction requires both Mg2+
Residue analysis of beta-naphthoxyacetic acid and beta-naphthol on field-sprayed tomatoes by high-pressure liquid chromatography.
T E Archer et al.
Journal of agricultural and food chemistry, 28(4), 877-880 (1980-07-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service