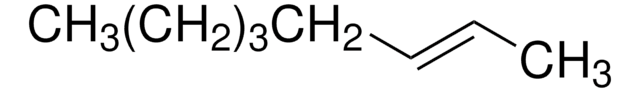All Photos(1)
About This Item
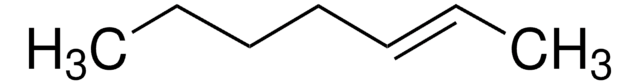
Linear Formula:
CH3CH2CH2CH=CHCH2CH2CH3
CAS Number:
Molecular Weight:
112.21
Beilstein:
1719104
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
Assay
≥90%
form
liquid
refractive index
n20/D 1.412 (lit.)
bp
122-123 °C (lit.)
solubility
water: insoluble
density
0.715 g/mL at 25 °C (lit.)
SMILES string
[H]\C(CCC)=C(\[H])CCC
InChI
1S/C8H16/c1-3-5-7-8-6-4-2/h7-8H,3-6H2,1-2H3/b8-7+
InChI key
IRUCBBFNLDIMIK-BQYQJAHWSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
trans-4-Octene is an acyclic olefin. Its synthesis has been reported. Its Raman spectra has been reported. Rate constant of the gas-phase reactions of trans-4-octene with hydroxyl radicals and ozone has been investigated. Its enantiomeric epoxidation has been studied. Its isomerization into isomeric octenes has been studied.
Application
trans-4-Octene may be used in the synthesis of the following:
- n-nonanal
- aliphatic unsaturated polyesters
- 4-isopropyloctane
- diasterioisomeric 4-(difluoroiodomethyl)-5-iodooctane
- siliranes
- m-dioxanes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
69.8 °F - closed cup
Flash Point(C)
21 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The Stereochemistry of the Debromination of Vicinal Dibromides by Metals1.
Schubert WM, et al.
Journal of the American Chemical Society, 74(18), 4590-4592 (1952)
Raman Spectra of Hydrocarbons I. 1-Octene, cis+ trans 2-Octene, trans-3-Octene, trans-4-Octene, 4-Octyne, and 1-Octyne.
Cleveland FF.
J. Chem. Phys. , 11(1), 1-6 (1943)
Synthesis and characterization of aliphatic unsaturated polyesters from trans-4-octene-1, 8-dioic and trans-3-hexene-1, 6-dioic acids.
Maglio G et al.
Eur. Polymer J., 15(7), 695-699 (1979)
A convenient, mild method for the cyclization of 3-and 4-arylalkanoic acids via their trifluoromethanesulfonic anhydride derivatives
Hulin B and Koreeda M.
The Journal of Organic Chemistry, 49(1), 207-209 (1984)
Efficient hydride-assisted isomerization of alkenes via rhodium catalysis.
Morrill TC and D'Souza CA.
Organometallics, 22(8), 1626-1629 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service