T1327
Tissue Inhibitor of Metalloproteinase-3 human
recombinant, expressed in NSO cells, >95% (SDS-PAGE)
Synonym(s):
TIMP-3
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
human
Quality Level
recombinant
expressed in NSO cells
Assay
>95% (SDS-PAGE)
form
lyophilized powder
mol wt
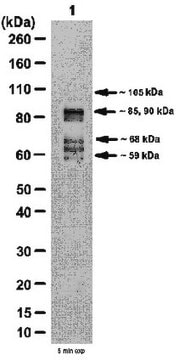
apparent mol wt ~30 kDa
technique(s)
inhibition assay: suitable
UniProt accession no.
storage temp.
−20°C
Gene Information
human ... TIMP3(7078)
Looking for similar products? Visit Product Comparison Guide
General description
Tissue inhibitor of metalloproteinases 3 (TIMP3) is localized in the extracellular matrix (ECM) of epithelial cells associated with kidneys, eyes and lungs. It is majorly secreted in retinal pigment epithelium (RPE). TIMP3 gene is mapped to human chromosome 22q12.3 and is a CLOCK-dependent diurnal gene. TIMP proteins display a three-lobed structure and have conserved cysteine residues.
Biochem/physiol Actions
Tissue inhibitor of metalloproteinases 3 (TIMP3) is a matrix metalloproteinase inhibitor. TIMP proteins comprise the N-terminal region, which aids in the matrix metalloproteinase interaction. The C-terminal region is crucial for extracellular matrix (ECM) interaction. Elevated expression of TIMP3 favors apoptosis in cancer types. Its interaction with the vascular endothelial growth factor (VEGFR-2) leads to the regulation of angiogenesis. TIMP3 also aids in protection against ultra-violet (UV)-induced cellular responses. Missense mutations in the TIMP3 gene are implicated in Sorsby′s fundus dystrophy (SFD). Mutations in the TIMP3 gene also leads to increased accumulation of the protein resulting in the thickening of the Bruch membrane. This, in turn, reduces membrane permeability towards nutrients and metabolites.
The TIMPs are endogenous inhibitors of the matrix metalloproteinases (MMPs). TIMP-3 inhibits ADAM-17 (TACE) at nanomolar concentrations and also inhibits other metalloproteinases (sheddases) that mediate the shedding of soluble receptors and other proteins from the surface of cells.
Physical form
Lyophilized from a 0.2 μm filtered solution in 25 mM Tris and 0.15 M sodium chloride, pH 7.5.
Analysis Note
The biological activity is measured by its ability to inhibit human MMP-2 hydrolysis of a peptide substrate.
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ruth Appeltant et al.
Animal science journal = Nihon chikusan Gakkaiho, 88(9), 1279-1290 (2017-01-27)
In vitro maturation (IVM) in serum causes hampered expansion of porcine cumulus-oocyte complexes (COCs) due to excessive alpha
Sunyoung Park et al.
International journal of molecular sciences, 20(4) (2019-02-20)
The human skin is the outermost physical barrier and has its own circadian machinery that works either cooperatively with the central clock, or autonomously. Circadian rhythms have been observed in many functions related to epidermal homeostasis including hydration and inflammation
Daniel Ardeljan et al.
European journal of human genetics : EJHG, 21(10), 1152-1157 (2013-02-21)
Age-related macular degeneration (AMD) is a leading cause of irreversible central visual loss in the elderly. A recent genome-wide association studies (GWAS) reported that rs9621532 near the tissue inhibitor of metalloproteinase 3 (TIMP3)/synapsin III (SYN3) region of 22q12.3 is associated
S M Silbiger et al.
Gene, 141(2), 293-297 (1994-04-20)
Proteins of the tissue inhibitor of metalloproteinase (TIMP) family bind and inactivate matrix metalloproteinases such as collagenases and gelatinases. We report the cloning and sequencing of cDNAs encoding a novel human TIMP, which we designated TIMP-3, the third member of
A Amour et al.
FEBS letters, 435(1), 39-44 (1998-10-02)
TNF-alpha converting enzyme (TACE; ADAM-17) is a membrane-bound disintegrin metalloproteinase that processes the membrane-associated cytokine proTNF-alpha to a soluble form. Because of its putative involvement in inflammatory diseases, TACE represents a significant target for the design of specific synthetic inhibitors
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service