77386
2-Bromo-1-ethyl-pyridinium tetrafluoroborate
≥97.0% (T)
Synonym(s):
BEP
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H9BBrF4N
CAS Number:
Molecular Weight:
273.86
Beilstein:
4059265
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0% (T)
form
crystals
reaction suitability
reaction type: Coupling Reactions
mp
103-107 °C
application(s)
peptide synthesis
functional group
bromo
SMILES string
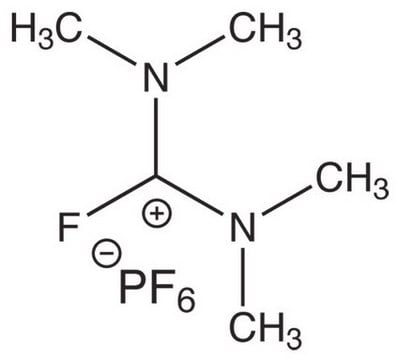
F[B-](F)(F)F.CC[n+]1ccccc1Br
InChI
1S/C7H9BrN.BF4/c1-2-9-6-4-3-5-7(9)8;2-1(3,4)5/h3-6H,2H2,1H3;/q+1;-1
InChI key
YJDXVQLBIAJTHP-UHFFFAOYSA-N
General description
2-Bromo-1-ethyl-pyridinium tetrafluoroborate is a coupling reagent employed in the synthesis of amides and esters through amidation and esterification reactions, respectively. It is generally prepared by the reaction of triethyloxonium tetrafluoroborate with 2?bromo pyridine.
Application
2-Bromo-1-ethyl-pyridinium tetrafluoroborate can be used as a coupling reagent for:
- The synthesis of N-methylated peptides in solution and solid phase.
- The synthesis of cyclosporin A fragment and dolastatin 15 pentapeptide moiety.
Other Notes
Coupling reagent for peptide synthesis; less racemization and faster reaction than with other reagents;e.g. BOP, PyBrOP, PyClU, BTFFH, CMBI
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Miguel J Xavier et al.
Reproduction (Cambridge, England), 156(3), 269-282 (2018-06-21)
The Big Blue λSelect-cII selection system has been employed along with whole-exome sequencing to examine the susceptibility of the male germ line to mutation in two challenging situations (i) exposure to a chemotherapeutic regime including bleomycin, etoposide and cis-platinum (BEP)
2?Bromo?1?ethyl Pyridinium Tetrafluoroborate (BEP)
Encyclopedia of Reagents for Organic Synthesis, Second Edition (2003)
1-Ethyl 2-halopyridinium salts, highly efficient coupling reagents for hindered peptide synthesis both in solution and the solid-phase
Li P and Xu J
Tetrahedron, 56(41), 8119-8131 (2000)
P Li et al.
The Journal of organic chemistry, 65(10), 2951-2958 (2000-05-18)
Cyclosporin O (1), an extensively N-methylated immunosuppressive cyclic undecapeptide isolated from Tolypocladium inflatum Gams, was synthesized in 20-23% overall yield via 4 + 7 segment condensation and cyclization by the combined utilization of novel thiazolium- and immonium-type peptide coupling reagents
Bernhard Banas et al.
BMC immunology, 18(1), 15-15 (2017-03-09)
Uncontrolled cytomegalovirus (CMV) replication in immunocompromised solid-organ transplant recipients is a clinically relevant issue and an indication of impaired CMV-specific cell-mediated immunity (CMI). Primary aim of this study was to assess the suitability of the immune monitoring tool T-Track® CMV
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service