731099
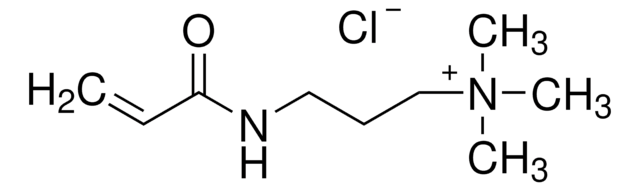
N-(3-Aminopropyl)methacrylamide hydrochloride
contains ≤1,000 ppm MEHQ as stabilizer, 98% (HPLC)
Synonym(s):
APMA
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C7H14N2O · HCl
CAS Number:
Molecular Weight:
178.66
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
98% (HPLC)
form
powder
contains
≤1,000 ppm MEHQ as stabilizer
mp
123-128 °C
storage temp.
2-8°C
SMILES string
Cl.CC(=C)C(=O)NCCCN
InChI
1S/C7H14N2O.ClH/c1-6(2)7(10)9-5-3-4-8;/h1,3-5,8H2,2H3,(H,9,10);1H
InChI key
XHIRWEVPYCTARV-UHFFFAOYSA-N
General description
N-(3-Aminopropyl)methacrylamide hydrochloride (APMA) is an aminoalkyl methacrylamide which can be synthesized by adding 1,3-diaminopropane to 1,3-diaminopropane dihydrogen chloride solution and further mixing the solution with methacrylic anhydride and hydroquinone. It has a primary amine that provides attractive features such as pH-responsiveness, affinity for anionic drugs and conjugation for variety of chemical structures.
Application
APMA can be used in the preparation of copolymers and cross-linked miscellas for gene delivery, drug delivery and diagnostics applications.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Gaurasundar M Conley et al.
Nature communications, 10(1), 2436-2436 (2019-06-06)
Thermosensitive microgels are widely studied hybrid systems combining properties of polymers and colloidal particles in a unique way. Due to their complex morphology, their interactions and packing, and consequentially the viscoelasticity of suspensions made from microgels, are still not fully
Facile synthesis of controlled-structure primary amine-based methacrylamide polymers via the reversible addition-fragmentation chain transfer process
Deng Z, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 46(15), 4984-4996 (2008)
Efficient RAFT polymerization of N-(3-aminopropyl) methacrylamide hydrochloride using unprotected ?clickable? chain transfer agents
Mendoncca PV, et al.
Reactive and Functional Polymers, 81(2), 1-7 (2014)
Preparation and characterization of narrow compositional distribution polyampholytes as potential biomaterials: Copolymers of N-(3-aminopropyl) methacrylamide hydrochloride (APM) and methacrylic acid (MAA)
Dubey A, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 53(2), 353-365 (2015)
Polymer mediated peptide immobilization onto amino-containing N-isopropylacrylamide-styrene core-shell particles
Rossi S, et al.
Colloid and Polymer Science, 282(3), 215-222 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service






![N-[3-(Dimethylamino)propyl]methacrylamide 99%, contains MEHQ as inhibitor](/deepweb/assets/sigmaaldrich/product/structures/295/145/6b4aae15-7cb5-4b7b-9c06-8e6d24e50951/640/6b4aae15-7cb5-4b7b-9c06-8e6d24e50951.png)

![[3-(Methacryloylamino)propyl]trimethylammonium chloride solution 50 wt. % in H2O](/deepweb/assets/sigmaaldrich/product/structures/189/736/089bc8ae-2a98-416d-9f9a-a0a510b6b828/640/089bc8ae-2a98-416d-9f9a-a0a510b6b828.png)