55083
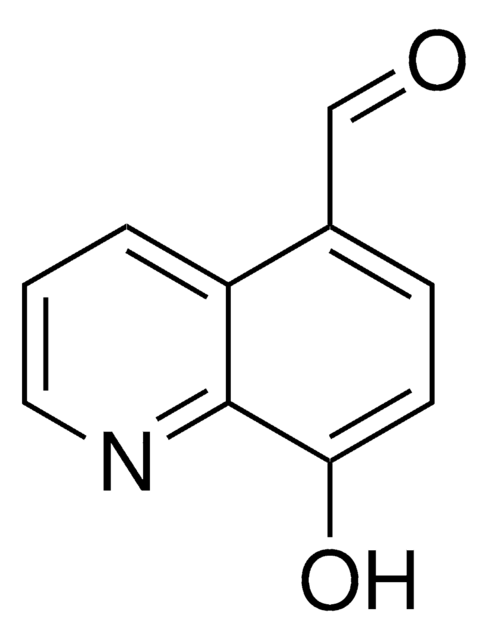
8-Hydroxy-2-quinolinecarboxaldehyde
≥98.0% (GC)
Synonym(s):
2-Formyl-8-hydroxyquinoline, 2-Formyl-8-quinolinol, 8-Hydroxyquinoline-2-aldehyde
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H7NO2
CAS Number:
Molecular Weight:
173.17
Beilstein:
127519
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (GC)
form
solid
mp
97-100 °C
functional group
aldehyde
SMILES string
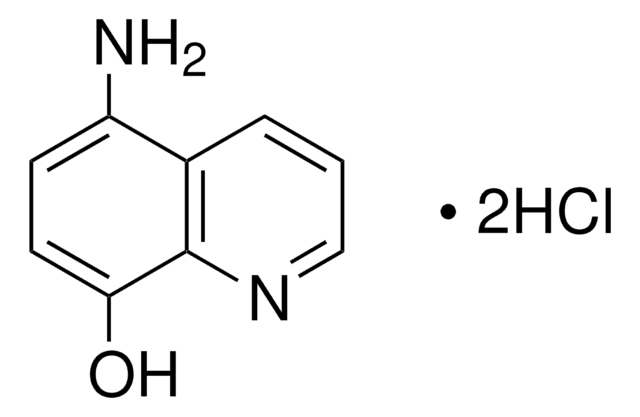
Oc1cccc2ccc(C=O)nc12
InChI
1S/C10H7NO2/c12-6-8-5-4-7-2-1-3-9(13)10(7)11-8/h1-6,13H
InChI key
SLBPIHCMXPQAIQ-UHFFFAOYSA-N
General description
8-Hydroxy-2-quinolinecarboxaldehyde can be prepared from 2-methylquinolin-8-ol via oxidation using selenium dioxide.
Application
8-Hydroxy-2-quinolinecarboxaldehyde (8-hydroxyquinoline-2-carbaldehyde) may be used in the preparation of:
- 8-hydroxy-2-quinoline-1-aminopyrene by Schiff-base reaction with 1-aminopyrene
- (E)-2-((2-(pyridin-2-yl)hydrazono)methyl)quinolin-8-ol by coupling with 2-hydrazinopyridine
- 8-hydroxyquinoline-2-carbaldehyde oxime
- 2-[(8-Hydroxyquinoline)-2-methylaminoethyl]-β-D-glucopyranoside
Other Notes
Building block for the synthesis of complexing agents
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
T. Hata et al.
Bulletin of the Chemical Society of Japan, 45, 477-477 (1972)
A. Yoneda et al.
Journal of Organometallic Chemistry, 401, 217-217 (1991)
Saptarshi Ghosh et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 188, 252-257 (2017-07-21)
The present work reports detailed photophysics of a coumarin based Schiff base, namely, (E)-7-(((8-hydroxyquinolin-2-yl)methylene)amino)-4-methyl-2H-chromen-2-one (HMC) in different solvents of varying polarity exploiting steady state absorption, fluorescence and time resolved fluorescence spectroscopy. The dominant photophysical features of HMC are discussed in
S. Sugata et al.
Chemical & Pharmaceutical Bulletin, 35, 2623-2623 (1987)
Rhombus-shaped tetranuclear [Ln4] complexes [Ln= Dy (III) and Ho (III)]: synthesis, structure, and SMM behavior.
Chandrasekhar V, et al.
Inorganic Chemistry, 52(11), 6346-6353 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service