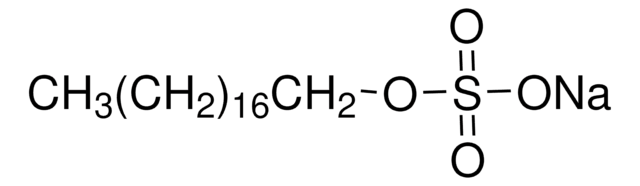318183
Methyl sulfate sodium salt
Synonym(s):
Monomethyl ester sulfuric acid sodium salt, Sodium methyl sulfate
About This Item
Recommended Products
form
powder
impurities
<3% methanol
<5% water
mp
210 °C (lit.)
SMILES string
[Na+].COS([O-])(=O)=O
InChI
1S/CH4O4S.Na/c1-5-6(2,3)4;/h1H3,(H,2,3,4);/q;+1/p-1
InChI key
DZXBHDRHRFLQCJ-UHFFFAOYSA-M
Application
- Methylsulfate anion based 1,3,4-trialkyl-1,2,3-triazolium ionic liquids for use in Morita–Baylis–Hillman reaction.
- Anisole by reacting with phenol.
- Hydroxyanilino quinolines for use as RET kinase inhibitors.
- Studying lamellar structure formation in hybrid nanomaterials created by miniemulsion
- EPR studies of radical ions radiolytically generated from ionic liquids, used as a reference
- Micellular studies specifically the rate-retarding effects on hydrolysis of substituted 1-benzoyl-1,2,4-triazoles and self-assembly / microstructure of mixed micelles
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service