S8876
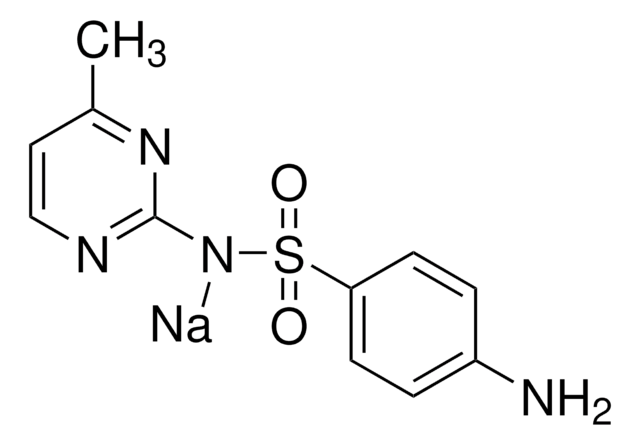
Sulfamerazine
ReagentPlus®, ≥99.0%
Synonym(s):
4-Amino-N-(4-methyl-2-pyrimidinyl)benzenesulfonamide, N1-(4-Methylpyrimidin-2-yl)sulfanilamide
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Empirical Formula (Hill Notation):
C11H12N4O2S
CAS Number:
Molecular Weight:
264.30
Beilstein:
249133
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Assay:
≥99.0%
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
≥99.0%
SMILES string
Cc1ccnc(NS(=O)(=O)c2ccc(N)cc2)n1
InChI
1S/C11H12N4O2S/c1-8-6-7-13-11(14-8)15-18(16,17)10-4-2-9(12)3-5-10/h2-7H,12H2,1H3,(H,13,14,15)
InChI key
QPPBRPIAZZHUNT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A practical sulfenylation of 2,5-diketopiperazines.
K C Nicolaou et al.
Angewandte Chemie (International ed. in English), 51(3), 728-732 (2011-12-14)
Yi Li et al.
International journal of pharmaceutics, 415(1-2), 110-118 (2011-06-08)
The ability to detect and quantify polymorphism of pharmaceuticals is critically important in ensuring that the formulated product delivers the desired therapeutic properties because different polymorphic forms of a drug exhibit different solubilities, stabilities and bioavailabilities. The purpose of this
Mehdi D Esrafili et al.
Journal of molecular graphics & modelling, 27(3), 326-331 (2008-07-08)
A density functional theory investigation was carried out to characterize (14)N electric field gradient tensors, EFG, in crystalline sulfamerazine and sulfathiazole. To include hydrogen-bonding effects in the calculations, the most probable interacting molecules with the target were considered as tetrameric
Florian M Koch et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 17(13), 3679-3692 (2011-03-03)
The first catalytic asymmetric synthesis of β-sultones is reported. This development has enabled a rapid access to a number of highly enantioenriched biologically interesting sulfonyl and sulfinyl compound classes, which makes use of the inherent ring strain of the four-membered
Lionel Billiet et al.
Free radical biology & medicine, 52(8), 1473-1485 (2012-02-14)
Protein sulfenic acids are essential cysteine oxidations in cellular signaling pathways. The thermodynamics that drive protein sulfenylation are not entirely clear. Experimentally, sulfenic acid reduction potentials are hard to measure, because of their highly reactive nature. We designed a calculation
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service