All Photos(1)
About This Item
Empirical Formula (Hill Notation):
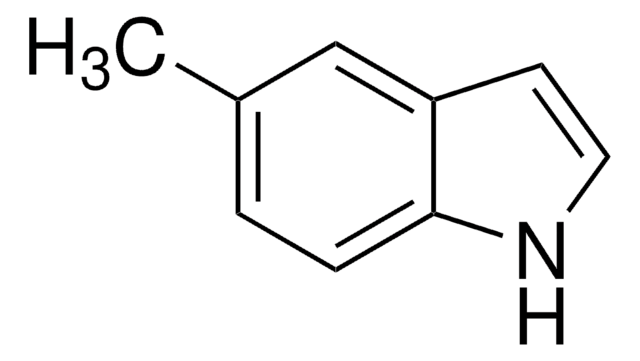
C9H9N
CAS Number:
Molecular Weight:
131.17
Beilstein:
109781
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
bp
273 °C (lit.)
mp
57-59 °C (lit.)
SMILES string
Cc1cc2ccccc2[nH]1
InChI
1S/C9H9N/c1-7-6-8-4-2-3-5-9(8)10-7/h2-6,10H,1H3
InChI key
BHNHHSOHWZKFOX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant for:
- Regioselective synthesis of oxopyrrolidine analogs via iodine-catalyzed Markovnikov addition reaction
- Friedel-Crafts alkylation reactions
- Preparation of tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Preparation of plant-growth inhibitors
- Michael addition reactions
- Synthesis of cyclooxygenase-1 (COX-1)/cyclooxygenase-2 (COX-2) inhibitors
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
285.8 °F
Flash Point(C)
141 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
J M Gutteridge
The International journal of biochemistry, 14(7), 649-653 (1982-01-01)
1. The thiobarbituric acid (TBA) reaction, widely applied to the detection of autoxidation in polyunsaturated fatty acids, can be used to measure free-radical damage to amino acids, carbohydrates and nucleic acids. 2. In all of these systems malondialdehyde (MDA) is
Nevin Erciyes et al.
Journal of colloid and interface science, 278(1), 91-95 (2004-08-18)
The adsorption of indole and its 2-methyl derivative from aqueous solutions onto cobalt(II)-carboxylated diaminoethane sporopollenin (CDAE-sporopollenin) was studied using a fixed-bed column at 25+/-0.1 degrees C. Minicolumn adsorption studies showed that the breakthrough and the total adsorption capacities of CDAE-sporopollenin
[2-Methylindoles substituted in the 1st, 3d and 5th positions and the diffuse neuroendocrine APUD system].
K S Shadurskiĭ et al.
Farmakologiia i toksikologiia, 46(2), 115-120 (1983-03-01)
V F Ximenes et al.
Archives of biochemistry and biophysics, 387(2), 173-179 (2001-05-24)
The indole moeity is present in many substances of biological occurrence. Its metabolism, in most cases, involves an oxidative pathway. This study reports the oxidation of a series of indole derivatives, including several of biological origin, catalyzed by horseradish peroxidase
N G Faleev et al.
Biochemistry and molecular biology international, 35(5), 1037-1040 (1995-04-01)
Tryptophanase was generally considered to be inactive towards tryptophan derivatives substituted at 2-position of the indole ring. We have shown that cells containing tryptophanase catalyze the formation of 2-methyl-L-tryptophan from 2-methylindole and L-serine, and from 2-methylindole, pyruvate and ammonium ion.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service