All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H5N3
CAS Number:
Molecular Weight:
95.10
Beilstein:
107025
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
form:
crystals
Assay:
98%
Recommended Products
Quality Level
Assay
98%
form
crystals
mp
118-120 °C (lit.)
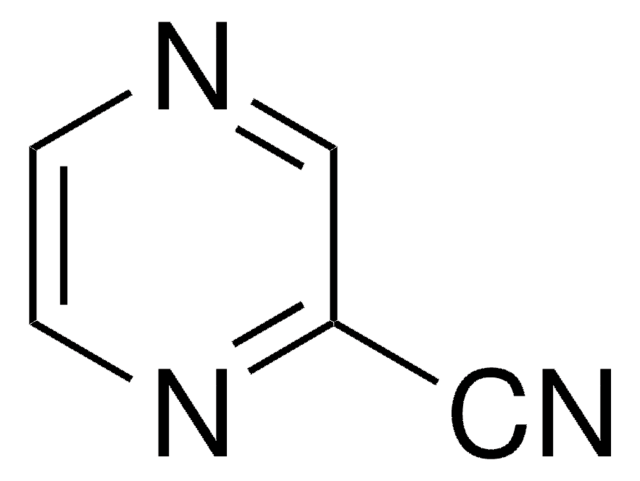
SMILES string
Nc1cnccn1
InChI
1S/C4H5N3/c5-4-3-6-1-2-7-4/h1-3H,(H2,5,7)
InChI key
XFTQRUTUGRCSGO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Substrate in a four-component synthesis of imidazolidines.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthetic Communications, 37, 247-247 (2007)
W H Lunn et al.
Xenobiotica; the fate of foreign compounds in biological systems, 22(11), 1239-1241 (1992-11-01)
1. The compound 2-aminopyrazine was given by oral gavage to normal rats and their urine collected. 2. A mercapturic acid containing the 2-aminopyrazine moiety was isolated from this urine. This represents the first example of this type of a metabolite
J F Cavalier et al.
Bioorganic & medicinal chemistry, 9(4), 1037-1044 (2001-05-17)
A series of 5-aryl- and 3,5-bis-aryl-2-amino-1,4-pyrazine derivatives 4 and 6, and related imidazolopyrazinones 7, has been synthesized, the aryl groups of which are catechol and/or phenol substituents. These compounds, tested against human keratinocyte cells stressed by UVB irradiation, showed high
Abdullah M Asiri et al.
Molecules (Basel, Switzerland), 12(8), 1796-1804 (2007-10-26)
New Schiff bases derived from 2-aminopyridene and 2-aminopyrazine have been synthesized. The UV-Visible spectra of the compounds have been investigated in acetonitrile and toluene. The compounds were in tautomeric equilibrium (enol-imine O- H...N, keto-amine O...H-N forms) in polar and nonpolar
Jan Zitko et al.
Molecules (Basel, Switzerland), 23(9) (2018-09-21)
Three series of N-(pyrazin-2-yl)benzamides were designed as retro-amide analogues of previously published N-phenylpyrazine-2-carboxamides with in vitro antimycobacterial activity. The synthesized retro-amides were evaluated for in vitro growth inhibiting activity against Mycobacterium tuberculosis H37Rv (Mtb), three non-tuberculous mycobacterial strains (M. avium
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service