All Photos(1)
About This Item
Empirical Formula (Hill Notation):
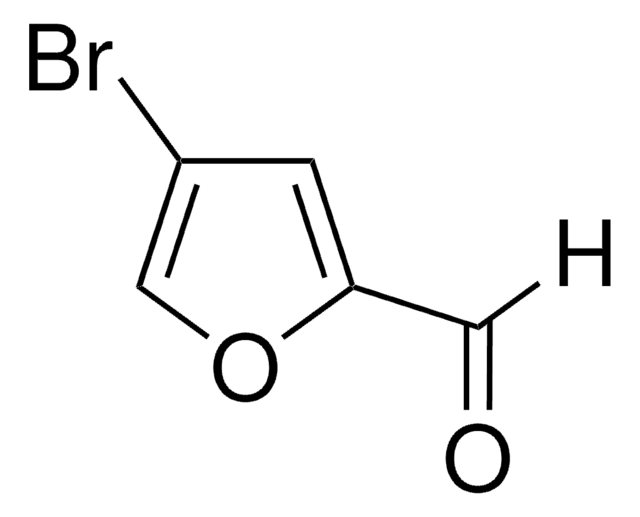
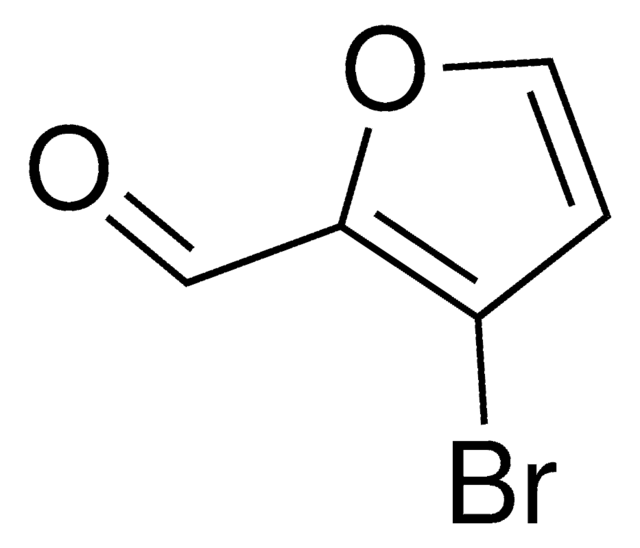
C5H3BrO2
CAS Number:
Molecular Weight:
174.98
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
bp
112 °C/16 mmHg (lit.)
mp
82-85 °C (lit.)
storage temp.
2-8°C
SMILES string
Brc1ccc(C=O)o1
InChI
1S/C5H3BrO2/c6-5-2-1-4(3-7)8-5/h1-3H
InChI key
WJTFHWXMITZNHS-UHFFFAOYSA-N
Application
5-Bromo-2-furaldehyde may be employed for the following syntheses:
- 5-substituted 2-furaldehydes
- anilines, through a novel one-pot, two-step amination/Diels-Alder procedure
- 5-(5′,8′-dimethyl-9′-tert-butoxycarbonyl-9′H-carbazol-3′-yl)-furan-2-carbaldehyde
- 5-(6-hydroxyhexyl)-2-furaldehyde
- 5-methylsulfonyl-2-furaldehyde
- 5-phenylsulfonyl-2-furaldehyde
- 5-(4-acetamidobenzylsulfonyl)-2-furaldehyde
- 5-iodo-2-furaldehyde
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A synthesis strategy yielding skeletally diverse small molecules combinatorially.
Burke MD, et al.
Journal of the American Chemical Society, 126(43), 14095-14104 (2004)
Efficient and Simple Synthesis of 6-Aryl-1,4-dimethyl-9H-carbazoles.
Caruso A, et al.
Molecules (Basel), 13(6), 1312-1320 (2008)
Furan derivatives. LXXXV11. The synthesis and ultraviolet spectra of 5-(4-X-phenyIsulfonyI)-2-furaldehydes and 2-cyano-3-[5-(4-X-phenyl-sulfonyl)-2-furyl] acrylonitriles.
Kada R and Kovac J.
Chemical Papers, 30(4), 502-507 (1976)
Photochemical coupling between halogenoheterocyclic and heterocyclic derivatives.
D'Agostini A and D'Auria M.
Journal of the Chemical Society. Perkin Transactions 1, 9, 1245-1249 (1994)
Efficient coupling of heteroaryl bromides with arylboronic acids in the presence of a palladium-tetraphosphine catalyst.
Feuerstein M, et al.
Tetrahedron Letters, 42(23), 5659-5662 (2001)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service