389439
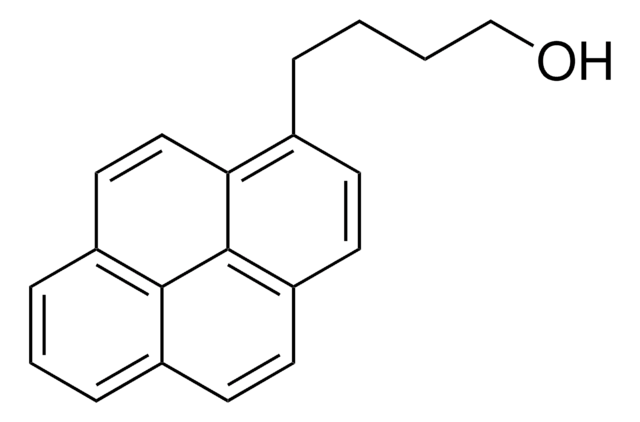
1-Pyrenemethanol
98%
Synonym(s):
1-(1-Hydroxymethyl)pyrene, 1-Hydroxymethylpyrene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C17H12O
CAS Number:
Molecular Weight:
232.28
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
123-126 °C (lit.)
functional group
hydroxyl
SMILES string
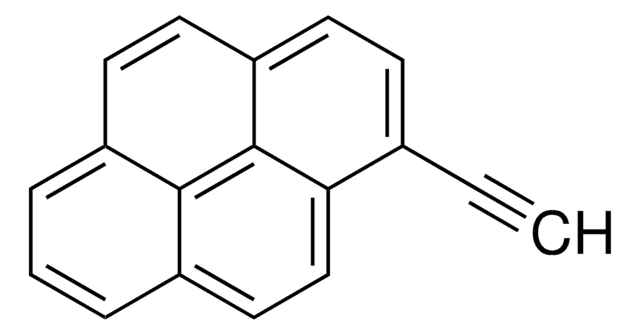
OCc1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C17H12O/c18-10-14-7-6-13-5-4-11-2-1-3-12-8-9-15(14)17(13)16(11)12/h1-9,18H,10H2
InChI key
NGDMLQSGYUCLDC-UHFFFAOYSA-N
Application
1-Pyrenemethanol can be used:
- For the synthesis of pincer-like benzene-bridged fluorescent selective sensor for adenosine-5′-triphosphate (ATP) detection.
- As a starting material for the synthesis of pyrene-end poly(glycidyl methacrylate) polymer.
- As an initiator for the synthesis of pyrene core star polymers.
- For the synthesis of 1-pyrenecarboxaldehyde, an important intermediate in pharmaceutical and agrochemical fields.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
G Werle-Schneider et al.
Carcinogenesis, 14(11), 2267-2270 (1993-11-01)
Rat liver cytosolic hydroxysteroid sulfotransferases form highly reactive sulfuric acid esters from some benzylic alcohols, such as 1-hydroxymethylpyrene. In this study we examined the expression of hydroxysteroid sulfotransferase a (STa) in carcinogen-induced enzyme-altered, presumably preneoplastic, rat liver foci. Female Wistar
Ronny Kollock et al.
Biochemical pharmacology, 75(2), 527-537 (2007-10-09)
Alkylated polycyclic aromatic hydrocarbons can be metabolically activated via benzylic hydroxylation and sulphation to electrophilically reactive esters. However, we previously found that the predominant biotransformation route for the hepatocarcinogen 1-hydroxymethylpyrene (1-HMP) in the rat in vivo is the oxidation of
H Glatt et al.
Environmental health perspectives, 88, 43-48 (1990-08-01)
Methylated polycyclic aromatic hydrocarbons are common in the human environment. Many of them are stronger carcinogens than their purely aromatic congeners. They may be metabolized to benzylic alcohols. We report here on biochemical and toxicological characteristics of 1-hydroxymethylpyrene (HMP), a
C D Sherman et al.
Carcinogenesis, 16(10), 2499-2506 (1995-10-01)
The promotional effect of phenobarbital and 1-hydroxymethyl-pyren on enzyme altered lesions in the rat liver were quantified within the framework of two separate multipath/multistage models. The experiment analyzed followed an initiation-promotion protocol in which female Wistar rats were initiated with
H Glatt et al.
Mutation research, 324(3), 111-114 (1994-07-01)
Previous studies demonstrated that the ion composition of the exposure medium may strongly influence the mutagenicity of many compounds in the liquid preincubation modification of the reversion assay with his- Salmonella typhimurium strains. Similar influences were now observed in the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service