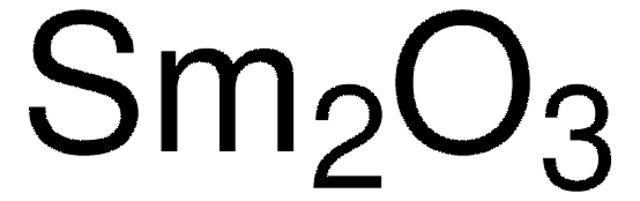228672
Samarium(III) oxide
99.9% trace metals basis
Synonym(s):
Samaria
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
Sm2O3
CAS Number:
EC Number:
MDL number:
UNSPSC Code:
12352303
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
99.9% trace metals basis
form
powder
reaction suitability
reagent type: catalyst
core: samarium
density
8.35 g/mL at 25 °C (lit.)
SMILES string
O=[Sm]O[Sm]=O
InChI
1S/3O.2Sm
InChI key
PRCWVHVINXAFRG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Lloyd G Tillman et al.
Journal of pharmaceutical sciences, 97(1), 225-236 (2007-08-28)
Treatment of systemic disease with phosphorothioate antisense oligonucleotides (PS ASOs) has been accomplished using local or parenteral routes of administration to date. This report describes, for the first time, the effective oral delivery of a second generation oligonucleotide where significant
Tuuli Marvola et al.
International journal of pharmaceutics, 349(1-2), 24-29 (2007-09-18)
The fate of two colon-specific formulations developed in our previous study was investigated using a gamma scintigraphic imaging method. The formulations contained paracetamol and samarium oxide (Sm2O3) and either microcrystalline cellulose (MCC) or hypromellose (HPMC K4M) as diluent and were
Yasunori Fukuda
Journal of radiation research, 43 Suppl, S67-S69 (2003-06-10)
In order to observe and estimate the dose of ultraviolet (UV) radiation, the thermoluminescence (TL) of sintered CaF2 doped with Tb4O7 and Sm2O3 was studied. A several kind of lanthanides elements are doped in pure CaF2 powder crystals and properties
H M T U Herath et al.
Journal of biomedical materials research. Part A, 94(1), 130-136 (2010-02-04)
The biocompatibility of natural samarium (III) oxide, which has previously been used for treatment in bone-related diseases was determined as a first step in its evaluation as a bone implant material. Assessment for 28 days using osteoblast-like cells revealed no
Matthew D Burke et al.
Pharmaceutical research, 24(4), 695-704 (2007-03-21)
To develop a robust radiolabeling technique to enable evaluation of difficult to radiolabel gastric retentive formulations using gamma scintigraphy. The use of a successful radiolabel will allow accurate assessment of the gastric residence time of the formulations. The retention of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service