106399
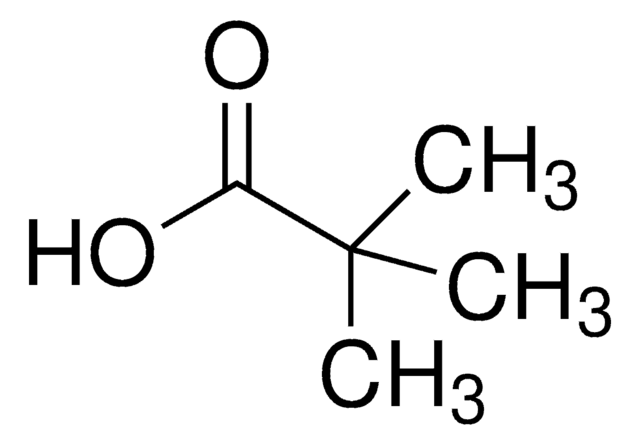
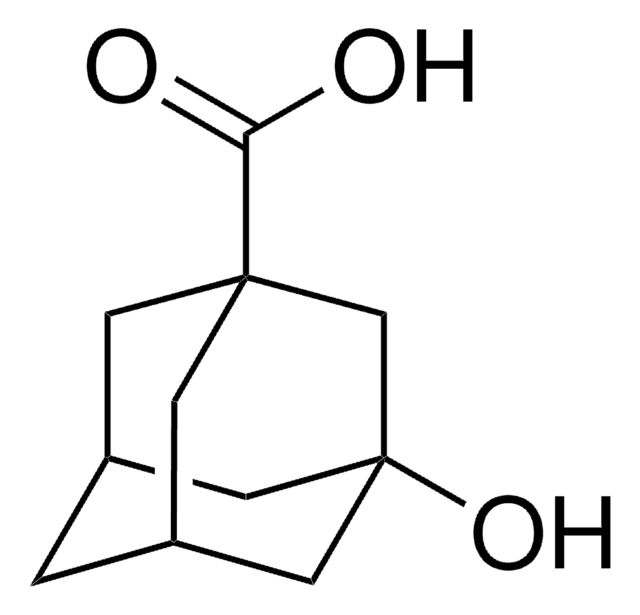
1-Adamantanecarboxylic acid
99%
Synonym(s):
Adamantane carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H16O2
CAS Number:
Molecular Weight:
180.24
Beilstein:
1910637
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
172-174 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)C12C[C@H]3C[C@H](C[C@H](C3)C1)C2
InChI
1S/C11H16O2/c12-10(13)11-4-7-1-8(5-11)3-9(2-7)6-11/h7-9H,1-6H2,(H,12,13)/t7-,8+,9-,11-
InChI key
JIMXXGFJRDUSRO-KJZNFTALSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
1-Adamantanecarboxylic acid can be used as:
- A stabilizer in the synthesis of monodisperse, highly crystalline CoPt3 nanoparticles and porous platinum nanoparticles.
- An additive in polycondensation reactions to yield conjugated polymers as possible optoelectronic materials.
- An additive in the allylic substitution reaction, which is catalyzed by palladium in an aqueous medium.
Biochem/physiol Actions
1-Adamantanecarboxylic acid undergoes complexation reactions with cyclohexaamylose. It is an inhibitor of phenyl ester hydrolysis of cycloheptaamylose.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Masaki Nakahata et al.
Nature communications, 2, 511-511 (2011-10-27)
Expanding the useful lifespan of materials is becoming highly desirable, and self-healing and self-repairing materials may become valuable commodities. The formation of supramolecular materials through host-guest interactions is a powerful method to create non-conventional materials. Here we report the formation
Thomas Le Saux et al.
Electrophoresis, 26(16), 3094-3104 (2005-07-26)
Among the different experimental strategies available in capillary electrophoresis (CE) to determine binding parameters, affinity capillary electrophoresis (ACE) has been the most widely embraced due to its easiness of implementation and of data handling. Ligand-substrate binding constants are thus directly
Birgit Hakkarainen et al.
Carbohydrate research, 340(8), 1539-1545 (2005-05-12)
The hydrogen-bond network in mono-altro-beta-cyclodextrin and in its inclusion complex with adamantane-1-carboxylic acid were investigated by (1)H NMR spectroscopy using the chemical shifts, temperature coefficients and vicinal coupling constants of the exchangeable hydroxy protons. The chemical shifts of the 3-OH
Detailed optimization of polycondensation reaction via direct C-H arylation of ethylenedioxythiophene
Yamazaki K, et al.
Macromolecular Rapid Communications, 34(1), 69-73 (2013)
Xiaopeng Han et al.
Journal of chromatographic science, 54(8), 1460-1465 (2016-06-01)
An efficient extraction of doxorubicin (Dox) from homemade stealth hyalurionic acid (HA)-based nanoparticles (NPs) in rat plasma could not be performed by previously published methods. Therefore, we attempted to establish the novel NPs-breaking and UPLC-MS-MS method for evaluating the pharmacokinetic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service