All Photos(1)
About This Item
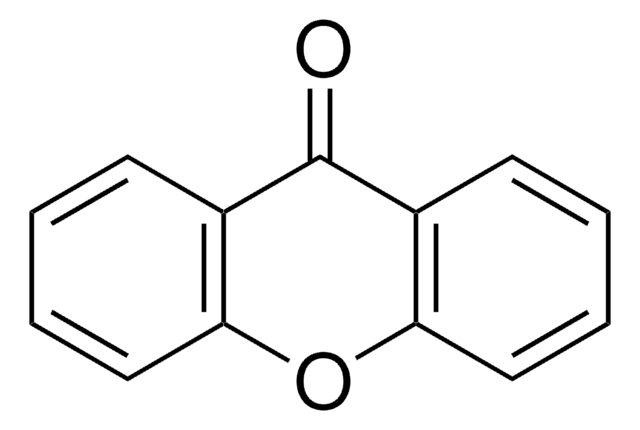
Empirical Formula (Hill Notation):
C13H8O2
CAS Number:
Molecular Weight:
196.20
Beilstein:
140443
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
bp
349-350 °C/730 mmHg (lit.)
mp
172-174 °C (lit.)
SMILES string
O=C1c2ccccc2Oc3ccccc13
InChI
1S/C13H8O2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1-8H
InChI key
JNELGWHKGNBSMD-UHFFFAOYSA-N
Gene Information
mouse ... Prkch(18755)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rajan Giri et al.
Bioorganic & medicinal chemistry, 18(4), 1456-1463 (2010-02-05)
A series of substituted xanthenes was synthesized and screened for activity using DU-145, MCF-7, and HeLa cancer cell growth inhibition assays. The most potent compound, 9 g ([N,N-diethyl]-9-hydroxy-9-(3-methoxyphenyl)-9H-xanthene-3-carboxamide), was found to inhibit cancer cell growth with IC(50) values ranging from
Ibrahim Jantan et al.
Phytochemistry, 80, 58-63 (2012-05-30)
Three benzophenones, 2,6,3',5'-tetrahydroxybenzophenone (1), 3,4,5,3',5'-pentahydroxybenzophenone (3) and 3,5,3',5'-tetrahydroxy-4-methoxybenzophenone (4), as well as a xanthone, 1,3,6-trihydroxy-5-methoxy-7-(3'-methyl-2'-oxo-but-3'-enyl)xanthone (9), were isolated from the twigs of Garcinia cantleyana var. cantleyana. Eight known compounds, 3,4,5,3'-tetrahydroxy benzophenone (2), 1,3,5-trihydroxyxanthone (5), 1,3,8-trihydroxyxanthone (6), 2,4,7-trihydroxyxanthone (7), 1,3,5,7-tetrahydroxyxanthone (8)
Yann Fromentin et al.
Organic letters, 14(19), 5054-5057 (2012-09-19)
Benzoylphloroglucinol derivatives are natural products showing diverse biological activities that could be modulated by structural modifications. For this purpose, we studied the biotransformation of guttiferone A and of maclurin using a combinatorial approach for screening active microorganism strains. We found
Kavitha Sudheendran et al.
The Journal of organic chemistry, 77(22), 10194-10210 (2012-10-17)
As little as 0.5 mol % CuCl is sufficient to catalyze the intramolecular O-arylation of easily accessible 2-(2-bromobenzyl)cyclohexane-1,3-diones to provide the corresponding 2,3,4,9-tetrahydro-1H-xanthen-1-ones with yields ranging from 83% to 99%.
Ruttiros Khonkarn et al.
Colloids and surfaces. B, Biointerfaces, 94, 266-273 (2012-03-02)
Xanthone exhibits several medicinal activities and especially it inhibits the growth of cancer cells. However, the use of xanthone is limited because of its low aqueous solubility and systemic toxicity. In the present study xanthone was loaded into poly(ethylene glycol)-b-poly[N-(2-hydroxypropyl)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service