286044
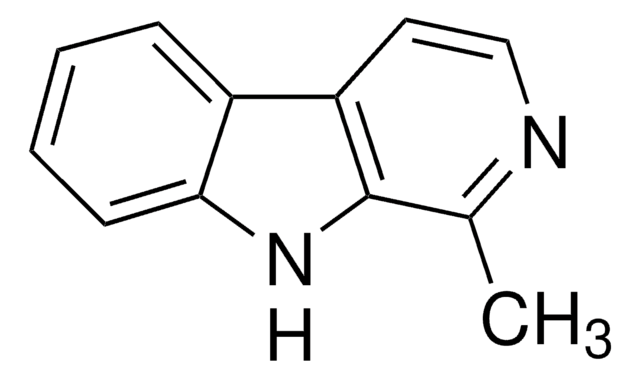
Harmine
98%
Synonym(s):
7-Methoxy-1-methyl-9H-pyrido[3,4-b]indole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C13H12N2O
CAS Number:
Molecular Weight:
212.25
Beilstein:
178813
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
262-264 °C (lit.)
SMILES string
CC1=NC=CC2=C1NC3=C2C=CC(OC)=C3
InChI
1S/C13H12N2O/c1-8-13-11(5-6-14-8)10-4-3-9(16-2)7-12(10)15-13/h3-7,15H,1-2H3
InChI key
BXNJHAXVSOCGBA-UHFFFAOYSA-N
Gene Information
human ... CDC2(983) , CYP2D6(1565)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
The combination index (CI, serves as a quantitative indicator of pharmacological interactions) for harmaline/harmine and methylene blue/harmine was studied.
Application
Harmine was used as a donor in the fluorescence resonance energy transfer (FRET) study between harmine and silver nanoparticles (AgNPs).
Biochem/physiol Actions
Central nervous system stimulant.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Katie L Uhl et al.
Cancer cell international, 18, 82-82 (2018-07-07)
Neuroblastoma (NB) is an early childhood malignancy that arises from the developing sympathetic nervous system. Harmine is a tricyclic β-carboline alkaloid isolated from the harmal plant that exhibits both cytostatic and cytotoxic effects. Harmine is capable of blocking the activities
Michael F Santillo et al.
Toxicology in vitro : an international journal published in association with BIBRA, 28(3), 403-410 (2014-01-01)
Interactions among monoamine oxidase (MAO) inhibitors in drugs, botanicals, and dietary supplements may lead to unpredictable neurochemical dysfunction due to excessive inhibition or therapeutic invalidation. Often recombinant MAO or brain tissue homogenates have been used to evaluate MAO inhibitors such
Mansour Debdab et al.
Journal of medicinal chemistry, 54(12), 4172-4186 (2011-05-28)
We here report on the synthesis, optimization, and biological characterization of leucettines, a family of kinase inhibitors derived from the marine sponge leucettamine B. Stepwise synthesis of analogues starting from the natural structure, guided by activity testing on eight purified
Mohammad Amjadi et al.
Luminescence : the journal of biological and chemical luminescence, 29(6), 689-694 (2013-11-30)
This article reports on a novel fluorescence resonance energy transfer (FRET) system between harmine and silver nanoparticles (AgNPs), in which harmine acts as the donor and AgNPs act as the acceptor. As a result of FRET, harmine fluorescence is quenched
Dea Shahinas et al.
Journal of medicinal chemistry, 53(9), 3552-3557 (2010-03-31)
Malaria is responsible for 3 million deaths annually. Antimalarial drug resistance is widespread, and few novel, well-defined targets exist. A robotic high throughput screen (HTS) was performed using 4000 small molecules from a natural compound (Spectrum), pharmacologically active (Lopac), and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service