115819
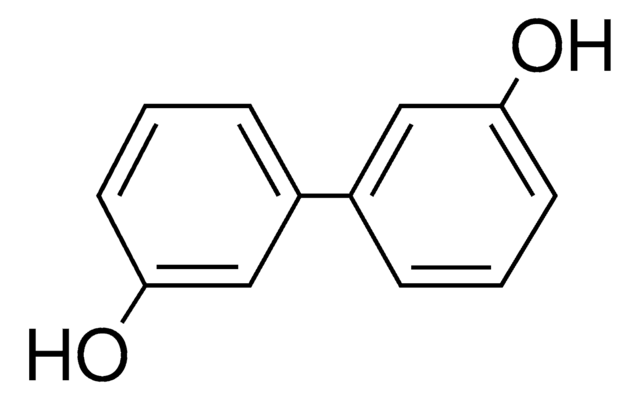
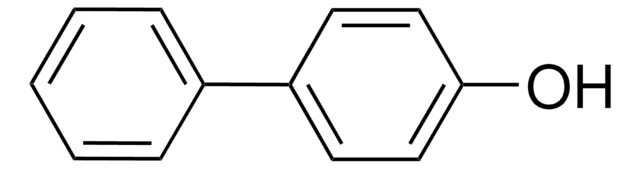
2,2′-Biphenol
99%
Synonym(s):
2,2′-Biphenyldiol, 2,2′-Dihydroxybiphenyl, 2,2′-Diphenol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H4C6H4OH
CAS Number:
Molecular Weight:
186.21
Beilstein:
1638363
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
99%
form
solid
bp
315 °C (lit.)
mp
108-110 °C (lit.)
SMILES string
Oc1ccccc1-c2ccccc2O
InChI
1S/C12H10O2/c13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)14/h1-8,13-14H
InChI key
IMHDGJOMLMDPJN-UHFFFAOYSA-N
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
309.2 °F - closed cup - (External MSDS)
Flash Point(C)
154 °C - closed cup - (External MSDS)
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
V V Subrahmanyam et al.
Xenobiotica; the fate of foreign compounds in biological systems, 20(12), 1369-1378 (1990-12-01)
1. 14C-Phenol was metabolized by rat bone marrow homogenate and H2O2. The homogenate catalyst, however, was inactivated by preincubation with H2O2, presumably due to inactivation of the enzyme(s) involved in phenol metabolism. 2. The majority of the metabolized 14C-phenol was
Zwe-Ling Kong et al.
Bioorganic & medicinal chemistry letters, 15(1), 163-166 (2004-12-08)
The neolignans, magnolol 1 and honokiol 2 have been reported to inhibit the growth of several tumor cell lines in vitro and in vivo. The chemical structure of magnolol and honokiol consists of biphenyl skeleton with phenolic and allylic functionalities.
Zengqi Xie et al.
Organic letters, 12(14), 3204-3207 (2010-06-22)
Facile nucleophilic substitution of two chlorine atoms by 2,2'-biphenol at one of the two bay areas (1,12- and 6,7-positions) of core-tetrachlorinated perylene bisimide afforded a novel, completely desymmetrized perylene bisimide building block, which could be further functionalized by substitution of
H P Kohler et al.
Journal of bacteriology, 175(6), 1621-1628 (1993-03-01)
Cells of Pseudomonas sp. strain HBP1 grown on 2-hydroxy- or 2,2'-dihydroxybiphenyl contain NADH-dependent monooxygenase activity that hydroxylates 2,2'-dihydroxybiphenyl. The product of this reaction was identified as 2,2',3-trihydroxybiphenyl by 1H nuclear magnetic resonance and mass spectrometry. Furthermore, the monooxygenase activity also
W A Prütz et al.
International journal of radiation biology and related studies in physics, chemistry, and medicine, 44(2), 183-196 (1983-08-01)
Phenoxyl radicals generated pulse radiolytically by the reaction of N.3 with Gly-Tyr decay biomolecularly (2k = 4.7 X 10(8)M-1 s-1) with efficient formation of 2,2'-dimers, which enolize rapidly (k = 2.7 X 10(4) s-1) to produce the 2,2'-biphenolic product. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service