L2037
β-Lapachone
≥98% (TLC)
Sinónimos:
ARQ 501, NSC 26326, NSC 629749, SL 11001
Seleccione un Tamaño
Seleccione un Tamaño
About This Item
Productos recomendados
origen biológico
synthetic (organic)
Ensayo
≥98% (TLC)
Formulario
powder
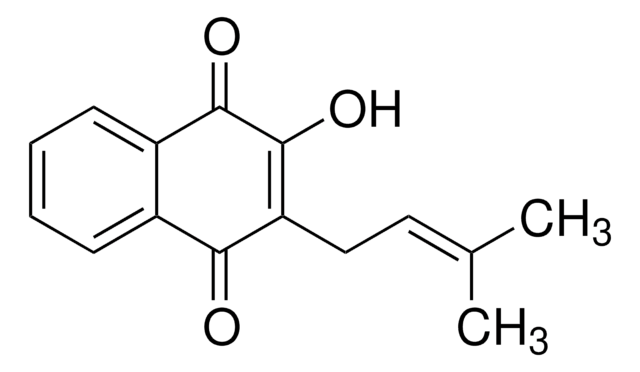
cadena SMILES
CC1(C)CCC2=C(O1)c3ccccc3C(=O)C2=O
InChI
1S/C15H14O3/c1-15(2)8-7-11-13(17)12(16)9-5-3-4-6-10(9)14(11)18-15/h3-6H,7-8H2,1-2H3
Clave InChI
QZPQTZZNNJUOLS-UHFFFAOYSA-N
Aplicación
- as an anticancer compound in catalase-inhibitable luminol/hydrogen peroxide (HRP)-dependent chemiluminometric assay in Lewis lung carcinoma (LLC) cells and isolated mitochondria[1]
- as a naphthoquinone to study its effects on the growth and differentiation of mice granulocyte and macrophage progenitor cells[2]
- as a substrate to study the enzyme activity of human recombinant NAD(P)H dehydrogenase 1 (NQO1) protein[3]
Acciones bioquímicas o fisiológicas
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico