K0879
Canamicina sulfate from Streptomyces kanamyceticus
powder, γ-irradiated
Sinónimos:
Canamicina A, Canamicina sulfate salt
About This Item
Productos recomendados
biological source
Streptomyces kanamyceticus
grade
for molecular biology
sterility
γ-irradiated
form
powder
potency
≥700 μg per mg
color
white to off-white
pH
6.5-8.5(1% solution)
antibiotic activity spectrum
Gram-negative bacteria
Gram-positive bacteria
mycoplasma
mode of action
protein synthesis | interferes
storage temp.
2-8°C
suitability
nonselective for Escherichia coli
nonselective for coliforms
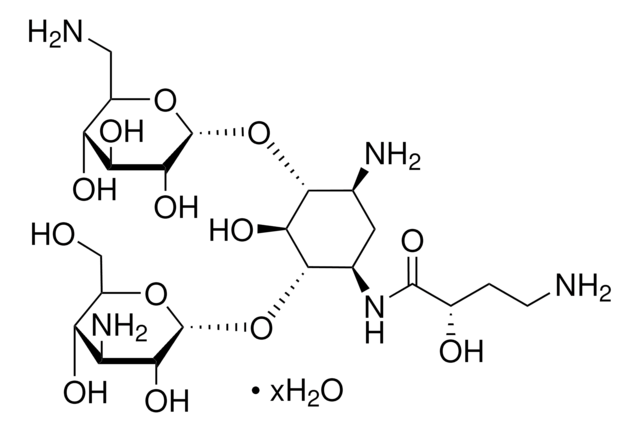
SMILES string
OS(O)(=O)=O.NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O[C@H]3O[C@H](CO)[C@@H](O)[C@H](N)[C@H]3O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C18H36N4O11.H2O4S/c19-2-6-10(25)12(27)13(28)18(30-6)33-16-5(21)1-4(20)15(14(16)29)32-17-11(26)8(22)9(24)7(3-23)31-17;1-5(2,3)4/h4-18,23-29H,1-3,19-22H2;(H2,1,2,3,4)/t4-,5+,6-,7-,8+,9-,10-,11-,12+,13-,14-,15+,16-,17-,18-;/m1./s1
InChI key
OOYGSFOGFJDDHP-KMCOLRRFSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
Biochem/physiol Actions
Modo de resistencia: las enzimas modificadoras de los aminoglucósidos (como la acetiltransferasa, la fosfotransferasa y la nucleotidiltransferasa) pueden alterar este antibiótico y evitar su interacción con los ribosomas.
Espectro antimicrobiano: El sulfato de canamicina es eficaz contra las bacterias gramnegativas y grampositivas, y contra los micoplasmas.
Principle
Preparation Note
Related product
signalword
Danger
hcodes
pcodes
Hazard Classifications
Repr. 1B
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 2
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico