H4398
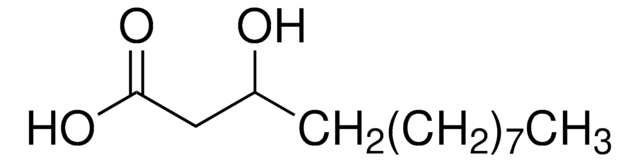
DL-β-Hydroxypalmitic acid
≥98%
Sinónimos:
3-Hydroxyhexadecanoic acid
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C16H32O3
Número de CAS:
Peso molecular:
272.42
Número MDL:
Código UNSPSC:
12352211
ID de la sustancia en PubChem:
NACRES:
NA.25
Productos recomendados
Ensayo
≥98%
Formulario
powder
grupo funcional
carboxylic acid
tipo de lípido
saturated FAs
Condiciones de envío
ambient
temp. de almacenamiento
2-8°C
cadena SMILES
CCCCCCCCCCCCCC(O)CC(O)=O
InChI
1S/C16H32O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-15(17)14-16(18)19/h15,17H,2-14H2,1H3,(H,18,19)
Clave InChI
CBWALJHXHCJYTE-UHFFFAOYSA-N
Categorías relacionadas
Acciones bioquímicas o fisiológicas
DL-β-Hydroxypalmitic acid is a mixture of D- and L-β-hydroxypalmitic (3-hydroxyhexadecanoic) acids. 3-hydroxyhexadecanoic [C16:0(3-OH)] may be used in studies that involve the lipid structures of endotoxin lipid A molecules.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
O Sebolai et al.
Antonie van Leeuwenhoek, 80(3-4), 311-315 (2002-02-06)
Electron microscopy studies indicated that the major oxylipin 3-hydroxy palmitic acid (16:0) was associated with aggregating vegetative cells and formed a web-like structure around these cells. Cross sections through this structure showed a hydrophilic outer layer and a more hydrophobic
David W Johnson et al.
Rapid communications in mass spectrometry : RCM, 17(2), 171-175 (2003-01-04)
Acetyl trimethylaminoethyl ester iodide derivatives have been used to selectively analyze isomeric long-chain hydroxy fatty acids by electrospray ionization tandem mass spectrometry (ESI-MS/MS). The binary derivatives of 2-, 3-, 12- and 16-hydroxypalmitic acids afford remarkably different product ion spectra. Further
M Fodorová et al.
Acta virologica, 55(1), 31-44 (2011-03-26)
Lipid A isolated from the Rickettsia typhi lipopolysaccharide (LPS) was investigated for its composition and structure using chemical analyses, gas chromatography-mass spectrometry (GC-MS), and electrospray ionization (ESI) combined with the tandem mass spectrometry (MS/MS). Our studies revealed a noticeable compositional
Anna Király et al.
Marine drugs, 11(12), 4858-4875 (2013-12-10)
The mechanism of action of elisidepsin (PM02734, Irvalec®) is assumed to involve membrane permeabilization via attacking lipid rafts and hydroxylated lipids. Here we investigate the role of hypoxia in the mechanism of action of elisidepsin. Culturing under hypoxic conditions increased
R P Garg et al.
Molecular microbiology, 38(2), 359-367 (2000-11-09)
In the phytopathogen Ralstonia (Pseudomonas) solanacearum, control of many virulence genes is partly mediated by the Phc cell density sensing system. Phc uses a novel self-produced signal molecule [3-hydroxypalmitic acid methyl ester (3-OH PAME)], an atypical two-component system (PhcS/PhcR), and
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico