A8326
Anti-β-Amyloid Protein (1-40) antibody produced in rabbit

whole antiserum
Sinónimos:
Anti-AAA, Anti-ABETA, Anti-ABPP, Anti-AD1, Anti-APPI, Anti-CTFgamma, Anti-CVAP, Anti-PN-II, Anti-PN2, Anti-alpha-sAPP, Anti-preA4
About This Item
Productos recomendados
biological source
rabbit
conjugate
unconjugated
antibody form
whole antiserum
antibody product type
primary antibodies
clone
polyclonal
form
liquid
contains
15 mM sodium azide
species reactivity
human
enhanced validation
independent
Learn more about Antibody Enhanced Validation
technique(s)
immunohistochemistry (formalin-fixed, paraffin-embedded sections): 1:100 using human Alzheimer’s disease (AD) brain tissue
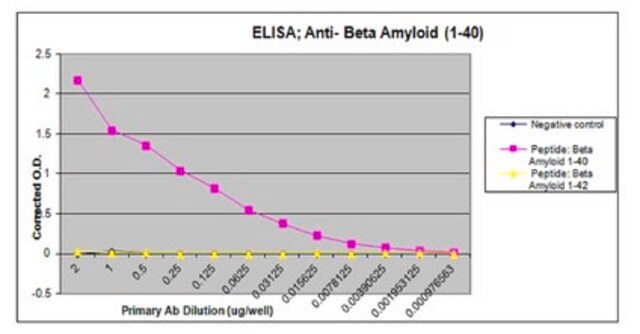
indirect ELISA: 1:4000-1:8000
UniProt accession no.
shipped in
dry ice
storage temp.
−20°C
target post-translational modification
unmodified
Gene Information
human ... APP(351)
Categorías relacionadas
General description
Immunogen
Application
- immunocytochemical localization of Aβ peptides
- immunocytochemistry
- immunoprecipitation
- focused ultrasound-microbubble enhanced antibody delivery (FUS-MB)
Biochem/physiol Actions
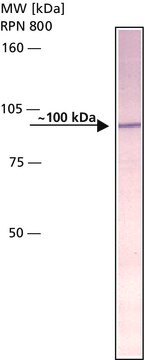
Rabbit Anti-β-Amyloid Protein (1-40) antibody does not stain control sections of normal brain tissues.
Physical form
Disclaimer
Not finding the right product?
Try our Herramienta de selección de productos.
related product
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico