64306
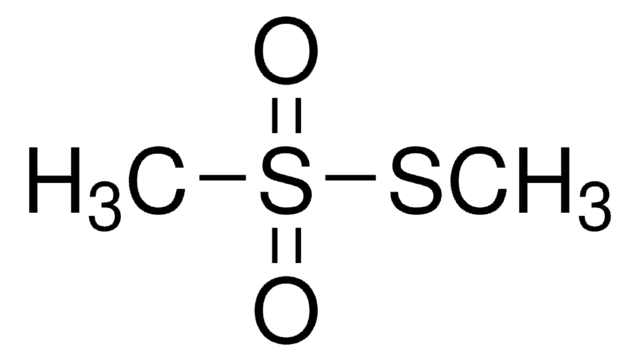
S-Methyl methanethiosulfonate
purum, ≥98.0% (GC)
Sinónimos:
S-Methyl thiomethanesulfonate, MMTS
About This Item
Productos recomendados
grade
purum
Quality Level
assay
≥98.0% (GC)
refractive index
n20/D 1.513 (lit.)
n20/D 1.513
bp
69-71 °C/0.4 mmHg (lit.)
solubility
chloroform: 750mg + 5 ml Chloroform mg/mL, colorless to light greenish-yellow
density
1.337 g/mL at 20 °C
1.337 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
CSS(C)(=O)=O
InChI
1S/C2H6O2S2/c1-5-6(2,3)4/h1-2H3
InChI key
XYONNSVDNIRXKZ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- Modification of Thiol Enzymes: S-methyl methanethiosulfonate (MMTS) offers a unique method for the modification of thiol enzymes and redox-regulated proteins, providing potential applications in biochemical research focused on enzyme regulation and redox biology (Makarov et al., 2019).
- Sensor Development for Protease Activity: S-methyl methanethiosulfonate is used as a blocking reagent on the structural transitions of papain-like cysteine proteases, which supports its utility in sensor development, allowing for the detection and analysis of protease activity in various biological processes (Markovic et al., 2023).
- Agricultural Pathogen Control: Research evaluating S-methyl methanethiosulfonate as a late blight inhibitor highlights its potential as a broad-range toxin against plant pathogens, suggesting applications in agriculture for the management of crop diseases (Joller et al., 2020).
Caution
Other Notes
Storage Class
10 - Combustible liquids
wgk_germany
WGK 2
flash_point_f
188.6 °F - closed cup
flash_point_c
87 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico