MM2PLTVP1
Milliflex Oasis® Customer Validation Protocol in letter format
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
UNSPSC Code:
41116500
NACRES:
NB.79
Productos recomendados
application(s)
bioburden testing
cosmetics
pharmaceutical
water monitoring
compatibility
for use with MILLIFLEX®
General description
Microbiological monitoring and testing in the pharmaceutical industry are highly regulated and a very complex field. In our long history of serving the pharmaceutical industry by pioneering and refining ground-breaking solutions, we have gained the regulatory and technical expertise to make compliance as simple as possible, and help save your valuable resources, by providing comprehensive range of professional and best-in-class services. Our Customer Validation Protocol makes validation faster, easier and ensures that your Milliflex Oasis® pump, accessories and related consumables do comply with the validated specifications. Our bioburden validation protocols follow international guidelines such as EP/USP and GMP.
With our best-in-class services you can:
With our best-in-class services you can:
- Optimize your QC lab workflow and ensure regulatory compliance
- Rely on comprehensive and ready-to-use validation packages
- Ensure the performance of your Milliflex Oasis® pump while reducing the risk of breakdown
Application
Our validation protocols are based on our internal product qualification test methods. These extensive protocols will enable the QC/QA lab to quickly initiate the validation master plan and perform IQ, OQ and PQ (suitability of the test methodology) with ease.
Features and Benefits
Count on our comprehensive and ready-to-use validation protocols consisting of the following sections:
1. Validation Master Plan: Define structure, responsibilities for qualification
2. Installation Qualification (IQ)
4. Performance Qualification (PQ) Test Method suitability verification (microbiology validation procedures)
5. Final Report Summarizes all testing performed for final approval of validation
1. Validation Master Plan: Define structure, responsibilities for qualification
2. Installation Qualification (IQ)
- Verification and identification of with your Milliflex Oasis® pump, accessories and related consumables
- Verification of product′s utilities and operating environment requirements
- Equipment and personnel preparation
4. Performance Qualification (PQ) Test Method suitability verification (microbiology validation procedures)
5. Final Report Summarizes all testing performed for final approval of validation
Legal Information
MILLIFLEX is a registered trademark of Merck KGaA, Darmstadt, Germany
MILLIFLEX OASIS is a registered trademark of Merck KGaA, Darmstadt, Germany
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
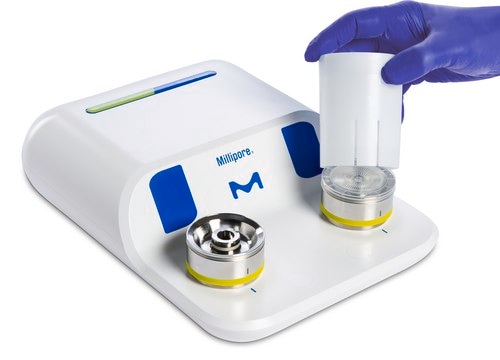
Milliflex Oasis® system monitors bioburden in various samples, increasing lab productivity.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico