218806
Caspase Inhibitor Set III
The Caspase Inhibitor Set III controls the biological activity of Caspase. This collection of small molecule/inhibitor is primarily used for Cancer applications.
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
UNSPSC Code:
12352200
Productos recomendados
Quality Level
form
liquid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
desiccated
shipped in
wet ice
storage temp.
−20°C
General description
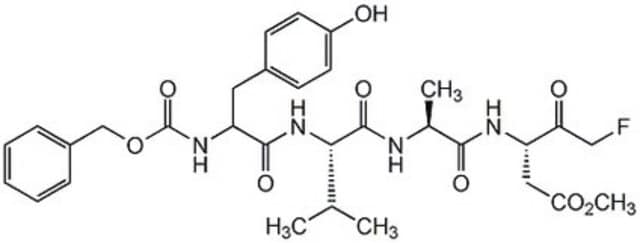
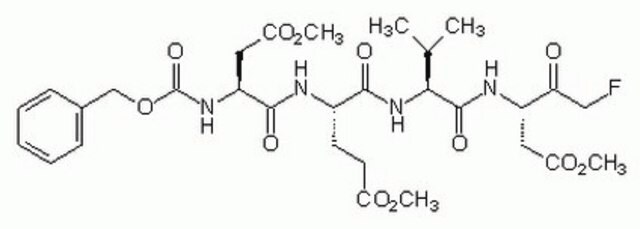
A set of eight ready to use cell-permeable, irreversible inhibitors of various caspase-family proteases. Contains 25 µl (2 mM) each of Caspase-1 Inhibitor VI, Z-YVAD-FMK (Cat. No. 218746); Caspase-2 Inhibitor I, Z-VDVAD-FMK (Cat. No. 218744); Caspase-3 Inhibitor II, Z-DEVD-FMK (Cat. No. 264155); Caspase-5 Inhibitor I, Z-WEHD-FMK (Cat. No. 218753); Caspase-6 Inhibitor I, Z-VEID-FMK (Cat. No. 218757); Caspase-8 Inhibitor II, Z-IETD-FMK (Cat. No. 218759); Caspase-9 Inhibitor I, Z-LEHD-FMK (Cat. No. 218761); and Caspase Inhibitor I, Z-VAD-FMK (Cat. No. 627610). Supplied with a data sheet.
Apoptosis is a normal process in development and morphogenesis. Many cells can be activated to undergo apoptosis following the interaction of selected ligands with cell surface receptors. Receptor-mediated apoptosis involves the activation of caspases (cysteine-containing aspartate-specific proteases). A distinctive feature of caspases is the requirement of an aspartic acid residue in the substrate P1 position.
Caspase inhibitors act by binding to the active site of caspases and form either a reversible or an irreversible linkage. Caspase inhibitor design includes a peptide recognition sequence attached to a functional group such as an aldehyde (CHO), chloromethylketone (CMK), fluoromethylketone (FMK), or fluoroacyloxymethylketone (FAOM). Caspase inhibitors with a CHO group are reversible and those with a CMK, FMK, or FAOM group are irreversible and cell-permeable. FMK exhibits slightly less reactivity than CMK and therefore is more specific for the enzyme being inhibited.
Caspase inhibitors act by binding to the active site of caspases and form either a reversible or an irreversible linkage. Caspase inhibitor design includes a peptide recognition sequence attached to a functional group such as an aldehyde (CHO), chloromethylketone (CMK), fluoromethylketone (FMK), or fluoroacyloxymethylketone (FAOM). Caspase inhibitors with a CHO group are reversible and those with a CMK, FMK, or FAOM group are irreversible and cell-permeable. FMK exhibits slightly less reactivity than CMK and therefore is more specific for the enzyme being inhibited.
Biochem/physiol Actions
Cell permeable: yes
Primary Target
Caspase-1, caspase-2, caspase-3, caspase-4, caspase-5, caspase-6, caspase-7, caspase-8
Caspase-1, caspase-2, caspase-3, caspase-4, caspase-5, caspase-6, caspase-7, caspase-8
Reversible: no
Warning
Toxicity: Irritant (B)
Physical form
Supplied as 2 mM in DMSO.
Other Notes
Humke, E.W., et al. 1998. J. Biol. Chem.273, 15702.
Datta, R., et al. 1997. J. Biol. Chem. 272, 1965.
Martin, L.M., et al. 1997. J. Biol. Chem. 272, 7421.
Takahashi, A., et al. 1997. Exp. Cell Res.231, 123.
Talanian, R.V., et al. 1997. J. Biol. Chem. 272, 9677.
Thornberry, N.A., et al. 1997. J. Biol. Chem. 272, 17907.
Nicholson, D.W., et al. 1995. Nature376, 37.
Thornberry, N.A., et al. 1992. Nature356, 768.
Datta, R., et al. 1997. J. Biol. Chem. 272, 1965.
Martin, L.M., et al. 1997. J. Biol. Chem. 272, 7421.
Takahashi, A., et al. 1997. Exp. Cell Res.231, 123.
Talanian, R.V., et al. 1997. J. Biol. Chem. 272, 9677.
Thornberry, N.A., et al. 1997. J. Biol. Chem. 272, 17907.
Nicholson, D.W., et al. 1995. Nature376, 37.
Thornberry, N.A., et al. 1992. Nature356, 768.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class
10 - Combustible liquids
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico