810226P
Avanti
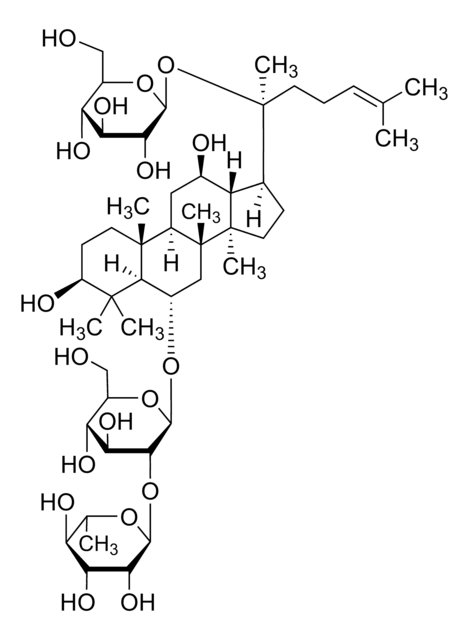
C6-NBD Lactosyl Ceramide
Avanti Research™ - A Croda Brand 810226P, powder
Sinónimos:
N-[6-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]hexanoyl]-D-lactosyl-β1-1′-sphingosine
Seleccione un Tamaño
Seleccione un Tamaño
About This Item
Productos recomendados
Ensayo
>99% (TLC)
Formulario
powder
envase
pkg of 1 × 1 mg (810226P-1MG)
pkg of 1 × 250 μg (810226P-250ug)
fabricante / nombre comercial
Avanti Research™ - A Croda Brand 810226P
Condiciones de envío
dry ice
temp. de almacenamiento
−20°C
Descripción general
Aplicación
- lactosylceramide synthase assay as a fluorescent acceptor substrate identified using fluorescence based detector in HPLC(1)
- phospholipid labeling and fluorescence-lifetime imaging microscopy (FILM) of cell membranes(6)
Acciones bioquímicas o fisiológicas
Envase
Información legal
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico