W237809
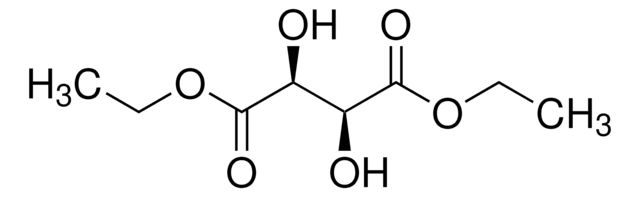
Diethyl L-tartrate
≥99%, FG
Sinónimos:
(+)-Diethyl L-tartrate, L-(+)-Tartaric acid diethyl ester
About This Item
Productos recomendados
biological source
synthetic
Quality Level
grade
FG
Fragrance grade
Kosher
agency
follows IFRA guidelines
meets purity specifications of JECFA
reg. compliance
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 117
FDA 21 CFR 172.515
assay
≥99%
optical activity
[α]20/D +8.5°, neat
refractive index
n20/D 1.446 (lit.)
bp
280 °C (lit.)
density
1.204 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
no known allergens
organoleptic
fruity; wine-like
SMILES string
CCOC(=O)[C@H](O)[C@@H](O)C(=O)OCC
InChI
1S/C8H14O6/c1-3-13-7(11)5(9)6(10)8(12)14-4-2/h5-6,9-10H,3-4H2,1-2H3/t5-,6-/m1/s1
InChI key
YSAVZVORKRDODB-PHDIDXHHSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
- Synthesis of l-threitol-based crown ethers and their application as enantioselective phase transfer catalyst in Michael additions.: This study synthesizes l-threitol-based crown ethers using diethyl ʟ-tartrate and explores their efficacy as enantioselective phase transfer catalysts in Michael additions, highlighting their potential in asymmetric synthesis (Rapi et al., 2017).
- A facile approach for the synthesis of C13-C24 fragments of maltepolides A, C and D.: This research demonstrates a novel synthesis method for C13-C24 fragments of maltepolides A, C, and D using diethyl ʟ-tartrate, facilitating the study and development of these bioactive compounds (Rao & Srihari, 2016).
- Development of diacyltetrol lipids as activators for the C1 domain of protein kinase C.: This research introduces diacyltetrol lipids synthesized from diethyl ʟ-tartrate, which act as activators for the C1 domain of protein kinase C, offering insights into signal transduction and therapeutic applications (Mamidi et al., 2012).
- Total synthesis of broussonetine F: the orthoamide Overman rearrangement of an allylic diol.: The paper presents the total synthesis of broussonetine F, utilizing diethyl ʟ-tartrate in an orthoamide Overman rearrangement, showcasing a novel synthetic route for complex natural products (Hama et al., 2011).
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
199.4 °F - closed cup
flash_point_c
93 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico