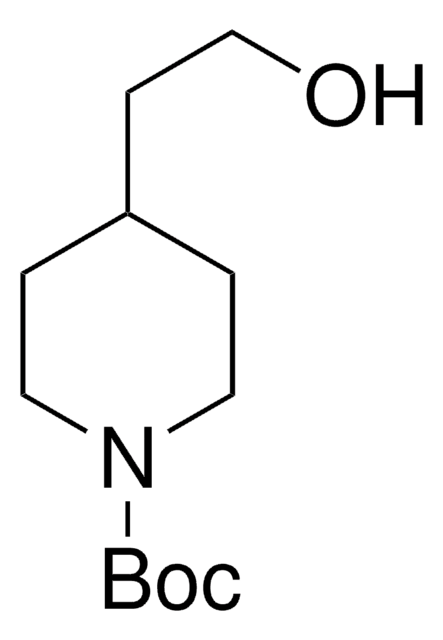
556017
N-Boc-4-piperidinemethanol
97%
Sinónimos:
N-tert-Butyloxycarbonyl-4-piperidinemethanol
About This Item
Productos recomendados
Ensayo
97%
Formulario
solid
mp
78-82 °C (lit.)
grupo funcional
hydroxyl
cadena SMILES
CC(C)(C)OC(=O)N1CCC(CO)CC1
InChI
1S/C11H21NO3/c1-11(2,3)15-10(14)12-6-4-9(8-13)5-7-12/h9,13H,4-8H2,1-3H3
Clave InChI
CTEDVGRUGMPBHE-UHFFFAOYSA-N
Descripción general
Código de clase de almacenamiento
13 - Non Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico