493937
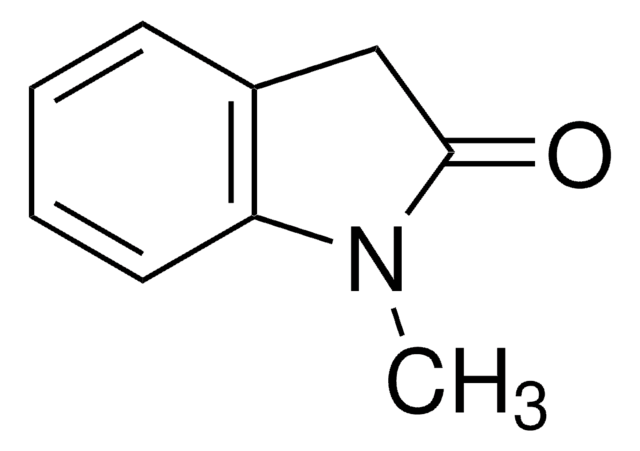
3-Methyl-2-oxindole
96%
Sinónimos:
3-Methyloxindole
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C9H9NO
Número de CAS:
Peso molecular:
147.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
96%
form
solid
mp
117-121 °C (lit.)
SMILES string
CC1C(=O)Nc2ccccc12
InChI
1S/C9H9NO/c1-6-7-4-2-3-5-8(7)10-9(6)11/h2-6H,1H3,(H,10,11)
InChI key
BBZCPUCZKLTAJQ-UHFFFAOYSA-N
Categorías relacionadas
General description
3-Methyl-2-oxindole (MOI) is a 3-substituted-2-oxindole. It is reported to be formed during the oxidation of indole-3-acetic acid in the presence of FeII under aerobic conditions. MOI undergoes asymmetric anti-Mannich-type reaction with N-tosyl aryl aldimines in the presence of alkaloid cinchona derivatives to form anti-3,3-disubsituted 2-oxindole derivatives. It also undergoes asymmetric hydroxyamination with nitrosoarenes to form N-nitroso aldol products.
Application
3-Methyl-2-oxindole may be used in the preparation of 3-hydroxy-3-methyl-2-oxindole.
- Reactant for enantioselective α-amination reactions
- Reactant for aldol reaction with glyoxal derivatives
- Reactant for amine thiourea catalyzed conjugate addition to α,β-unsaturated aldehydes
- Reactant for O-acetylation reactions
- Reactant for preparation of a disubstituted oxoindole by using rhodium-catalyzed cyclopropanation/ring-opening reactions
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
J Thornton-Manning et al.
The Journal of pharmacology and experimental therapeutics, 276(1), 21-29 (1996-01-01)
The toxicity of 3-methylindole (3 MI), a selective pneumotoxin, is dependent upon cytochrome P450-mediated bioactivation 3. Using vaccinia-expressed P450 enzymes, the metabolites of radiolabeled 3 MI produced by 14 individual P450s were identified and quantified by high performance liquid chromatography.
Jaroslav Matal et al.
Neuro endocrinology letters, 30 Suppl 1, 36-40 (2009-12-23)
To study the contribution of individual purified porcine CYP1A2, 2E1 and 2A19 enzymes to the biotransformation of skatole. Individual porcine and human enzymes (CYP1A2, 2E1 or 2A6/19) were used to study their potential involvement in skatole metabolism. Furthermore, the inhibition
Facile and Efficient Enantioselective Hydroxyamination Reaction: Synthesis of 3-Hydroxyamino-2-Oxindoles Using Nitrosoarenes.
Shen K, et al.
Angewandte Chemie (Weinheim an der Bergstrasse, Germany), 123(20), 4780-4784 (2011)
Ying Jin et al.
Chirality, 26(12), 801-805 (2014-07-22)
A series of cinchona alkaloid derivatives were used to catalyze the asymmetric anti-Mannich-type reaction of 3-methyl-2-oxindole with N-tosyl aryl aldimines. The resulting anti-3,3-disubstituted 2-oxindole products were obtained in good yields (up to 92%) with high diastereo- and enantioselectivities (anti/syn up
Metabolism and pneumotoxicity of 3-methyloxindole, indole-3-carbinol, and 3-methylindole in goats.
M J Potchoiba et al.
American journal of veterinary research, 43(8), 1418-1423 (1982-08-01)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico