423807
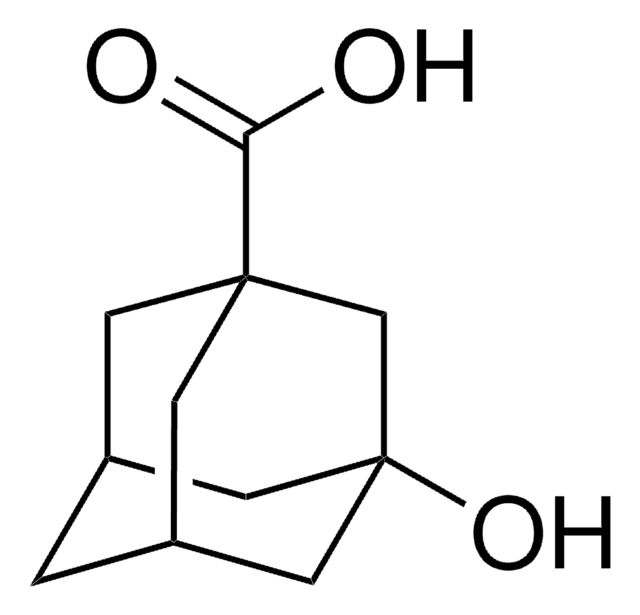
5-Hydroxy-2-adamantanone
98%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C10H14O2
Número de CAS:
Peso molecular:
166.22
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
98%
form
solid
mp
>300 °C (lit.)
SMILES string
O[C@]12C[C@@H]3C[C@H](C1)C(=O)[C@@H](C3)C2
InChI
1S/C10H14O2/c11-9-7-1-6-2-8(9)5-10(12,3-6)4-7/h6-8,12H,1-5H2/t6-,7-,8+,10-
InChI key
TZBDEVBNMSLVKT-XYYXLIQBSA-N
General description
5-Hydroxy-2-adamantanone is a disubstituted derivative of adamantane. The biocatalyzed synthesis of 5-hydroxy-2-adamantanone from 2-adamantanone has been investigated.
Application
5-Hydroxy-2-adamantanone may be used in the following studies:
- As a model compound to investigate the application of lanthanide NMR shift reagents for the analysis of disubstituted derivative of adamantane.
- As a starting material for the synthesis of E-2-amino-5-hydroxyadamantane.
- As a starting material for the synthesis of 4-(triphenylsilyloxy)adamantan-1-ol.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
S S Boĭko et al.
Eksperimental'naia i klinicheskaia farmakologiia, 57(6), 48-50 (1994-11-01)
The pharmacokinetics of the new immunostimulant kemantane, adamantane derivative, used in two species of animals (rats and rabbits) and man was studied. There were significant differences in the pharmacokinetics of kemantane and its active metabolite--adamantane-1,4-diol between the species.
Biocatalytic production of 5-hydroxy-2-adamantanone by P450cam coupled with NADH regeneration.
Furuya T, et al.
Journal of Molecular Catalysis. B, Enzymatic, 94, 111-118 (2013)
[The immunomodulator kemantan in the treatment of patients with exacerbated chronic obstructive bronchitis].
E M Rekalova
Likars'ka sprava, (4)(4), 73-76 (1992-04-01)
S S Boĭko et al.
Farmakologiia i toksikologiia, 54(1), 57-59 (1991-01-01)
The pharmacokinetics of a new Soviet-made immunostimulant kemantane, a derivative of adamantine, was studied by gas-liquid chromatography in patients with bronchial pathology. It was found that in the blood of the patients kemantane was not practically detected due to a
An expeditious preparation of E-2-amino-5-hydroxyadamantane and its Z-isomer.
Jaroskova L, et al.
Tetrahedron Letters, 47(46), 8063-8067 (2006)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico