380210
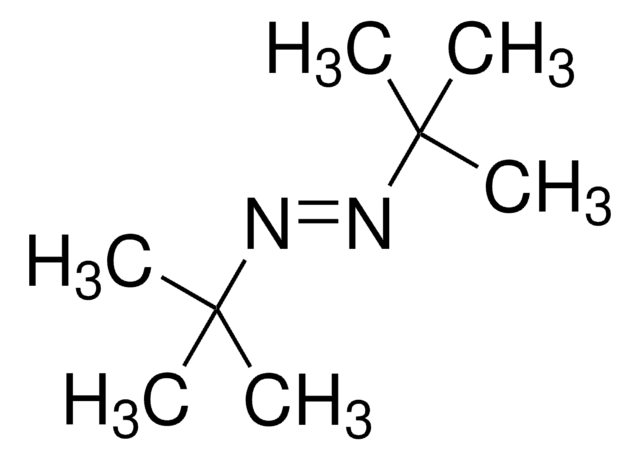
1,1′-Azobis(cyclohexanecarbonitrile)
98%
Sinónimos:
1,1′-Azobis(cyanocyclohexane), ACHN
About This Item
Productos recomendados
Quality Level
assay
98%
form
solid
mp
114-118 °C (lit.)
storage temp.
2-8°C
SMILES string
N#CC1(CCCCC1)\N=N\C2(CCCCC2)C#N
InChI
1S/C14H20N4/c15-11-13(7-3-1-4-8-13)17-18-14(12-16)9-5-2-6-10-14/h1-10H2/b18-17+
InChI key
KYIKRXIYLAGAKQ-ISLYRVAYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Self-react. D - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
5.2 - Organic peroxides and self-reacting hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
RAFT polymerization offers living characteristics to radical polymerization, contributing versatility to reversible deactivation radical polymerization methods.
Micro review of reversible addition/fragmentation chain transfer (RAFT) polymerization.
Protocolos
We presents an article featuring procedures that describe polymerization of methyl methacrylate and vinyl acetate homopolymers and a block copolymer as performed by researchers at CSIRO.
We present an article about RAFT, or Reversible Addition/Fragmentation Chain Transfer, which is a form of living radical polymerization.
Polymerization via ATRP procedures demonstrated by Prof. Dave Haddleton's research group at the University of Warwick.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico