300764
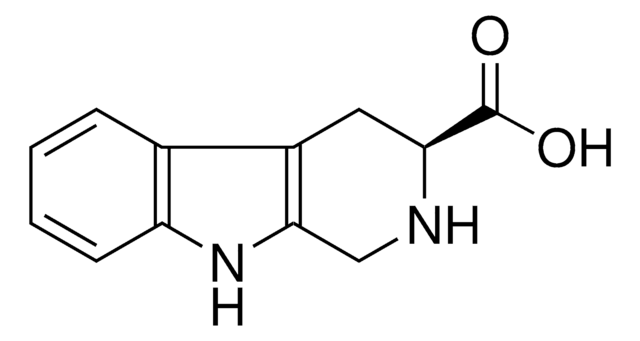
1,2,3,4-Tetrahydro-9H-pyrido[3,4-b]indole
98%
Sinónimos:
Noreleagnine, THBC, Tetrahydronorharman, Tryptoline
Seleccione un Tamaño
$39.80
Precio de catálogo$41.90Ahorre 5 %Disponible para envío el28 de marzo de 2025Detalles
Seleccione un Tamaño
About This Item
$39.80
Precio de catálogo$41.90Ahorre 5 %Disponible para envío el28 de marzo de 2025Detalles
Productos recomendados
Ensayo
98%
Formulario
liquid
mp
206-208 °C (lit.)
cadena SMILES
C1Cc2c(CN1)[nH]c3ccccc23
InChI
1S/C11H12N2/c1-2-4-10-8(3-1)9-5-6-12-7-11(9)13-10/h1-4,12-13H,5-7H2
Clave InChI
CFTOTSJVQRFXOF-UHFFFAOYSA-N
Información sobre el gen
rat ... Htr2a(29595) , Htr2c(25187)
Descripción general
Aplicación
- Reactant for synthesis of the indolyl-β-carboline alkaloid eudistomin U via IBX mediated room temperature oxidative aromatization
- Reactant for preparation of neuroprotective HDAC6 inhibitors
- Reactant for preparation of aminofuranopyrimidines as EGFR and Aurora A kinase inhibitors
- Reactant for preparation of inhibitors of CDK4
- Reactant for preparation of tetrahydrocarboline derivatives of as human 5-HT5A receptor ligands
- Reactant for preparation of 5-(diaminomethyl)-2,4-aminopyrimidines as dihydrofolate reductase inhibitors and antibacterial agents
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico


![2,3,4,5-Tetrahydro-1H-pyrido[4,3-b]indole AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/376/664/07577eb6-6e8c-4237-b8c5-03da4c8e7d88/640/07577eb6-6e8c-4237-b8c5-03da4c8e7d88.png)