223662
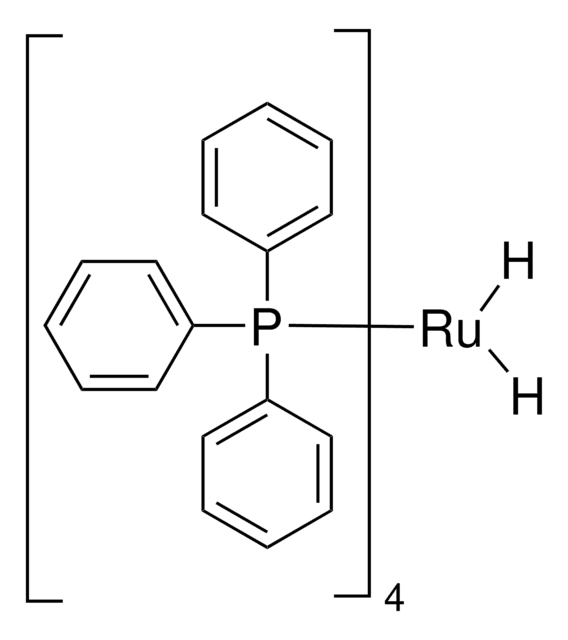
Tris(triphenylphosphine)ruthenium(II) dichloride
97%
Sinónimos:
Tris(triphenylphosphine)dichlororuthenium, Dichlorotris(triphenylphosphine)ruthenium(II), Ruthenium(II)-tris(triphenylphosphine) dichloride
About This Item
Productos recomendados
assay
97%
reaction suitability
core: ruthenium
reagent type: catalyst
reaction type: C-H Activation
SMILES string
Cl[Ru]Cl.c1ccc(cc1)P(c2ccccc2)c3ccccc3.c4ccc(cc4)P(c5ccccc5)c6ccccc6.c7ccc(cc7)P(c8ccccc8)c9ccccc9
InChI
1S/3C18H15P.2ClH.Ru/c3*1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;;;/h3*1-15H;2*1H;/q;;;;;+2/p-2
InChI key
WIWBLJMBLGWSIN-UHFFFAOYSA-L
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- Functionalized alcohols by C-C cross-coupling reaction between different alcohols via sp3 C-H bond activation of primary alcohols in the presence of Lewis acid.
- Furan derivatives from allenyl sulfides via 1,4 migration of the sulfanyl group.
- 1,3-diphenylpropan-1-one by alkylation of acetophenone with benzyl alcohol via C-C bond formation.
- Vinyl chloride monomer by hydrochlorination reaction of acetylene.
RuCl2(PPh3)3 can also be used as a catalyst in the cyclization, isomerization, reduction, oxidation, and cross-coupling reactions of a variety of organic products. Hydrogenation of nitro groups, imines, and ketones, as well as selective oxidation of alcohols are also possible in the presence of this catalyst.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico