KitAlysis™ High-Throughput Copper C-N Cross-Coupling Reaction Screening Kit
TABLE OF CONTENTS
Kit Design
Cu C-N (Buchwald-Hartwig) cross-coupling kit was designed to provide the best possible chance of success and is run with:
- 10 µmol aryl halide
- 15 µmol amine (primary, secondary), use 20 µmol if the material is not too precious
- 4 µmol ligand per vial (1:4, Cu:Ligand); 6 in this system (40 mol%)
- 30 µmol base (K3PO4)
- 0.1 M solvent concentration (Dioxane, DMAc, Toluene, & DMSO)
- 1 µmol CuI (soluble source, 10 mol%)
Use of the Provided Tools
Multiple tools have been created to ensure your success with kit set up. Start with the more detailed guide to ensure you are comfortable with all of the steps before using the quick guides on the excel worksheet. Remember that while the technique is new, it is still organic chemistry and so the steps will seem easy once you try just one kit. It is just a new way of approaching something you are already very good at.
Detailed Set-Up User Guide:
Designed for the first-time user and should be read completely before getting started. Best if used in conjunction with the video as not all steps are outlined in the video in great detail. This guide includes trouble-shooting tips, how-to’s for the Labware, and work-up recipes with procedures. Everything you need to set up a kit with confidence every time.
Excel Sheet:
Each sheet is designed to be used with the specific experimental design chosen by you depending upon the attributes of your substrates. The downloadable excel files are specific to the kit being run and can be found within each Step-by-Step User Guide. They have the following features:
Materials Included in your KITALYSIS-CN-2PK High-Throughput Screening Kit
Contents in each of the 2 individually sealed Mylar (foil bags):
- 24 (6 x 4) pre-weighed ligands in glass vials loaded with stir bars, topped with cap mat.
The screening sets come pre-loaded with 4 µmol of ligand in each vial according to the following design.

Figure 1.KitAlysis™ C-N Well Plate
Ampule Boxes
- Dioxane: 4 x 2 mL of degassed, anhydrous Aldrich® Sure/Seal™
- DMAc: 4 x 2 mL of degassed, anhydrous Aldrich® Sure/Seal™
- Toluene: 4 x 2 mL degassed anhydrous Aldrich® Sure/Seal™
- DMSO: 4 x 2 mL degassed anhydrous Aldrich® Sure/Seal™
Internal Standard
- Biphenyl (30 mg). To be added to the reaction during the work-up. Recipe for the work-up with the internal standard can be found within the hyperlinked screen types in the "Kit Design" section below.
KitAlysis™ 24-well Reaction Block Replacement Films-2EA
- Pack of 2 enables a new film to be used with each kit ensuring a tight, cross-contaminate-free seal every time.
Stir Bars-8 Individually Packed
- Fit perfectly into supplied vials to ensure proper stirring of substrate mixtures.
Additional Recommended Materials (Sold Separately)
- Z688711: 96-well plate for automated HPLC analysis
- C1111: 96-well plate cap mat for automated HPLC analysis
- Z683809: 10-100 uL pipette
- Z640220: 2-200 uL pipette tip refill
- Z683825: 100-1000 uL pipette
- Z640247: 50-1000 uL pipette tip refill
- Z192570: 2 inch needles for ease in ampule solvent extraction
- Z230723: 1 mL needle for accurate solvent volume extraction from ampule
Figure 2.
Materials Required - Set up
- 1 mylar bag from the KitAlysis™ Cu C-N Cross-Coupling Reaction Screening Kit and you will use the following components
- 6 x 4 pre-weighed ligands in glass vials loaded with stir bars and topped with cap mat
- 4, 4 mL reaction vials with pre-weighed K3PO4 base
- 1 pre-weighed vial of soluble CuI - 1 NEW KitAlysis™ 24-Well Reaction Block Replacement Film
- 1 (2mL) ampule each of Toluene, Dioxane, DMSO, and DMAc (in ampule boxes)
- 4 NEW stir bars
- KitAlysis™ 24-Well Reaction Block (sold separately)
- KitAlysis™ Benchtop Inertion Box (sold separately) or glove box/glove bag
- KitAlysis™ Torque Screwdriver Set (sold separately). Provided with the KitAlysis™ 24-Well Reaction Block
Additional (not included) items needed:
- Pipette (0-100 µL) & tips
- 4 (1 mL) syringes with long needles
- Your (het)aryl halide and amine
- Nitrogen (or Argon): from hood line or tank
- 1 hot plate with stir capabilities
- 1 stir plate (or hot plate to be used without heat)
- HPLC vials, 96-well HPLC auto sampler block, or TLC plates
Set Up Procedure
- Preheat a hot plate to 100 °C. It is highly recommended to use an oil bath or second reaction block to hold temperature and avoid spiking. Reaction temperatures for the Cu CN reaction can range from room temperature to 120 °C but 100 °C is a general and very good starting point.
- Place a NEW KitAlysis™ 24-Well Reaction Block Replacement Film on reaction block lid and verify all holes, including the temperature probe hole, line up with the corresponding holes on the film.
- Check all screws to ensure they are not stripped. Replace any stripped screws with provided replacements.
- Place the KitAlysis™ Benchtop Inertion Box with tubing connected to inert gas onto the second, non-heated stir plate (see KitAlysis Benchtop Inertion Box set-up for details)
- Place KitAlysis™ 24-Well Reaction Block with lid into KitAlysis™ Benchtop Inertion Box. Start nitrogen flow and purge 5 minutes. Leave nitrogen flowing for remainder of set up.
- Weigh both substrates directly into all of the 4 mL reaction vials (that contain the pre-weighed K3PO4) according to recipe (provided in the downloadable excel file) omitting solvent. Add one stir bar to each vial mixture. Label as “DMAc Substrate Mixture A”, “Toluene Substrate Mixture B”, “Dioxane Substrate Mixture C”, and “DMSO Mixture D”).
- Partially open the lid on the KitAlysis™ Benchtop Inertion Box. Place the “DMAc Substrate Mixture A” and the “Toluene Substrate Mixture B” in two of the holes located on the left hand side of the Inertion Box diffuser tray. Place the “Dioxane Substrate Mixture C”, and “DMSO Substrate Mixture D” in two holes on the right hand side of the plate. Take the vial of CuI from the mylar bag and place into the bottom right vial hole in the Inertion box – NOTE there will be no stir bar added to the CuI vial. (remove lids from the solid mixtures before placing them into the recommended holes, keeping the lids in the Inertion Box for later use if needed). Ensure that one vial is placed in the center hole on either side. This vial placement allows for the best flow of inert gas.
- Transfer the capped, 24-vial, preloaded catalysts into the reaction block making sure to load it according to the matching diagram on the packaging and the Reaction Block. Leave the mat on.
- Place 1 ampule each of DMAc, Toluene, Dioxane, and DMSO into ampule holes located along the bottom of the Inertion Box, below the Reaction Block.
- Once all components are in the KitAlysis™ Benchtop Inertion Box, close the lid and purge for an additional 5 minutes. Leave nitrogen flowing for remainder of set up.
- Using ampule cracker, open all ampules while still resting in the holes in the Inertion box (keeping them in the box under the flow of nitrogen).
- Purge needle and syringe in a nitrogen diffuser hole 2x by pulling and then pushing plunger. Using purged needle and syringe
- Add required solvent amounts to open substrate mixtures and CuI vials following recipe according to the excel file.
- Stir mixtures until in solution (1-2 min). For slurries, see “additional tips” below (there is no stir bar in the CuI but will go into solution with a little shake of the vial).
- While mixtures are stirring, carefully remove the cap mat from the 24-vial, preloaded catalysts in the reaction block.
- Dose stock solutions
- Dose 100 µL of “DMAc Substrate Mixture A” to vials A1-A6 according to scheme below.
- Dose 100 µL “Toluene Substrate Mixture B” into vials B1-B6 according to scheme below.
- Dose 100 µL “Dioxane Substrate Mixture C”, to vials C1-C6 according to scheme below.
- Dose 100 µL and “DMSO Substrate Mixture D” into vials D1-D6 according to scheme below.
You may have a very small amount of excess solution remaining for each mixture. Save it as a reaction standard for HPLC/TLC later.
- Dose 20 uL of the CuI solution to each of the 24 reaction vials A1-D6. It is helpful to say the vial location to yourself as you go along to keep track (A1, A2, etc).

- After all substrate mixtures and base have been dosed according to recipe, take the KitAlysis™ 24-Well Reaction Block lid and line up the screws with the holes in the plate. Ensure the temperature probe holes line up on both the lid and the block.
- Screw on lid according to directions and pattern shown in the “additional tips” section below. Before removing KitAlysis™ 24-Well Reaction Block from the Inertion Box, ensure that the lid is evenly sealed onto the base. Do this visual check as you go along to avoid having to unscrew the lid and screw again.
- Once completely sealed, remove the KitAlysis™ 24-Well Reaction Block from the Inertion Box. Place on preheated hot plate with probe through the lid and inserted into the block. Heat at 100 °C overnight stirring at or near 300 rpm. Higher or lower temperatures may be optimal, but 100 °C is a good starting point.
- Make the quench solution in a bottle with a replaceable lid following the recipe below for HPLC analysis. TLC is also possible.
- At reaction completion, follow the below Work-Up Procedure
Quench Solution Recipe
- 49 mL CH3CN
- 1 mL AcOH
- 15.4 mg Biphenyl (KitAlysis™ Internal Standard provided in the kit)
Note: This recipe makes 50 mL which is enough stock solution for all four screening sets in the KitAlysis™ Suzuki-Miyaura Cross-Coupling Reaction Screening Kit. The amount of internal standard is 10 mol%. So a big product peak to small internal standard indicates a good reaction.
Work-up Procedure and Analysis
- Cool Reaction Block. Remove lid using small, non-torque KitAlysis screwdriver.
- Check each vial for solvent loss, and record.
- Aliquot 500 µL of prepared quench solution to each vial.
- Replace lid, tighten middle screw and stir on stir plate (NO HEAT) for 2-3 minutes. DO NOT INVERT BLOCK.
- After 2-3 minutes of stirring, let plate rest (without stirring) for 5 minutes to allow insoluble material to settle out of solution to the bottom of the vials.
- While plate is resting, add 700 µL of acetonitrile to each 24 individually labeled (A1, A2 etc,) HPLC vials or to each of 24 wells of a 96-well HPLC/UPLC auto sampler block (see “additional recommended materials” below for suggestions on the auto sampler block and cap mat.
- Remove lid on KitAlysis™ 24-Well Reaction Block carefully.
- Using a clean pipette each time, remove a 25 µL aliquot from each vial into corresponding HPLC vials or HPLC block. Be careful to pull material from the top of the vials to avoid any precipitate.
- Run on HPLC auto sampler. You may need to adjust the amount of acetonitrile from the suggested 700 µL to accommodate your unique HPLC system.
Additional Tips
Sealing the Plate-screwing down the cover
Sealing the plate properly is critical to success. The key is light, even pressure to the lid of the block to keep the cover flat while sealing.
- Line-up screws making sure that the base and lid temperature probe holes line-up in the KitAlysis 24-Well Reaction Block.
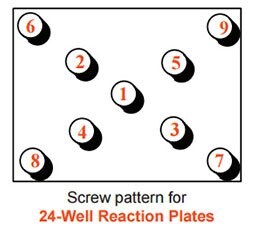
- Initially flush: Using your thumb and forefinger, press the lid of the box until it becomes flush with the vials. Then, using the KitAlysis Torque Screwdriver, insert screws until flush, but not tight, with the top of the box, following the cross pattern provided below. Check to see that the lid is evenly sealed onto the base on all sides. Do this visual check to avoid having to unscrew the lid and screw again.
- Tighten: Repeat the same pattern until the KitAlysis Torque Screwdriver “clicks” indicating complete tightness. Go around the block once more for a final check to ensure all screws are tight.
Probe for hot plate does not fit into reaction block hole
Use a small oil bath or other metal block (for best results) in the back of the hot plate and place the probe in there. Place reaction block as close to the center of the hot plate as possible for more even stirring.
Slurry Additions
Due to narrow opening of pipette tips, slurry additions will result in blockages. To aid in uniform dosing, simply snip off the end of the tip at the first marker (~10 mm). To ensure an evenly dispersed aliquot, it is critical that the mixture is stirring well while you draw aliquots for dosing into the corresponding reaction vial.

To continue reading please sign in or create an account.
Don't Have An Account?