Selecting a GC Column by a Specific Stationary Phase
Stationary Phase
Choosing a stationary phase is the most important step in choosing a column, and should be selected based on the application to be performed. It is recommended to first consult our Selecting a GC Column for Specific Industries/Applications section to determine if we have already identified appropriate columns. For new applications, there is often no existing reference to provide guidance. In these method development instances, one must have some knowledge of the chemistry of the compounds to be analyzed. Phase selection is based on the general chemical principle that "likes dissolves like" and relates to the specific analyte-stationary phase interactions that each group of columns can perform. Choose:
- Non-polar GC columns for non-polar compounds (such as alkanes) that contain 1) only carbon and hydrogen atoms, and 2) only single bonds between carbon atoms.
- Intermediate polar GC columns for an alternate selectivity of non-polar and/or polar compounds.
- Polar GC columns for polar compounds (such as alcohols, amines, carboxylic acids, diols, esters, ethers, ketones, and thiols) that contain 1) primarily carbon and hydrogen atoms, and 2) also some bromine, chlorine, fluorine, nitrogen, oxygen, phosphorus, and/or sulfur atoms.
- Highly polar GC columns for polarizable compounds (such as alkenes, alkynes, and aromatic hydrocarbons) that contain 1) only carbon and hydrogen atoms, and 2) some double and/or triple bonds between carbon atoms.
- Extremely polar GC columns for additional selectivity of polarizable compounds.
Non-Polar
Non-polar GC columns are made with the least selective of the GC stationary phases. They are commonly used to separate non-polar compounds (such as alkanes) that contain 1) only carbon and hydrogen atoms, and 2) only single bonds between carbon atoms. Elution order generally follows the boiling points of the analytes.
- Interactions are primarily dispersive (van der Waals forces).
- Phases with phenyl functional groups can also undergo a moderate amount of p-p interactions.
- PTA-5 columns are specially-engineered to also allow strong basic interactions.
- Phases with octyl functional groups also possess shape selectivity.
- Petrocol® DH Octyl, SPB®-Octyl, Petrocol® DH 50.2, Petrocol® DH, Petrocol® DH 150, Petrocol® 2887, Petrocol® EX2887, SLB®-1ms, SPB®-1 SULFUR, Equity®-1, SPB®-1, SLB®-5ms, MET-Biodiesel, SPB®-Biodiesel, MET-SimDis, HT-5 (aluminum clad), PTA-5, SAC™-5, Equity®-5, SPB®-5

Intermediate Polarity
Intermediate polar GC columns are made with phases that incorporate both non-polar and polar elements. Thus, they are commonly used to provide alternate selectivity to non-polar and polar columns. Elution order is determined by differences in the overall effects of possible interactions.
- Interactions are strongly dispersive (van der Waals forces). The greater the phenyl content of the phase, the stronger the interactions.
- Phases with phenyl functional groups can also undergo p-p, dipole-dipole, and dipole-induced dipole interactions. The greater the phenyl content, the stronger these interactions.
- Phases with cyanopropyl functional groups can also undergo strong dipole-dipole and moderate basic interactions. The greater the cyanopropyl content, the greater these interactions.
- SPB®-624, OVI-G43, VOCOL®, SPB®-20, Equity®-1701, SLB®-35ms, SPB®-608, Sup-Herb™, SPB®-35, SPB®-50, SP®-2250

Polar
Polar GC columns are made using polar stationary phases, the most common being polyethylene glycol and modified versions. These columns are commonly used to separate polar analytes (such as alcohols, amines, carboxylic acids, diols, esters, ethers, ketones, and thiols) that contain 1) primarily carbon and hydrogen atoms, and 2) also some bromine, chlorine, fluorine, nitrogen, oxygen, phosphorus, and/or sulfur atoms. Elution order is determined by differences in the overall effects of possible interactions.
- Dispersive (van der Waals forces), p-p, dipole-dipole, and dipole-induced dipole interactions are all strong with these columns.
- Moderate amounts of hydrogen bonding and basic interactions are also possible.
- SPB®-1000 and Nukol™ columns are specially-engineered to also allow strong acidic interactions.
- Carbowax® amine columns are specially-engineered to also allow strong basic interactions.
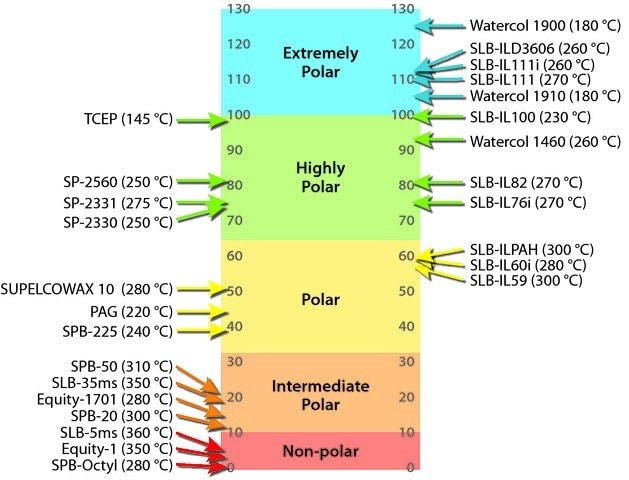
- SPB®-225, SPB®-PUFA, PAG, SPB®-1000, Nukol™, Carbowax® Amine, Omegawax®, SUPELCOWAX® 10, SLB®-ILPAH, SLB®-IL59, SLB®-IL60, SLB®-IL61

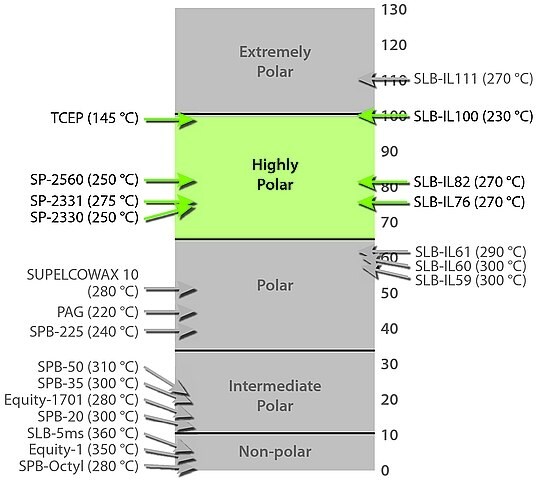
Highly Polar
Highly polar GC columns are made with very selective GC stationary phases, typically containing high percentages of cyanopropyl functional groups. They are commonly used to analyze polarizable compounds (such as alkenes, alkynes, and aromatic hydrocarbons) that contain 1) only carbon and hydrogen atoms, and 2) some double and/or triple bonds between carbon atoms. Elution order is determined by differences in the overall effects of possible interactions.
- Strong dispersive (van der Waals forces), very strong dipole-dipole, very strong dipole-induced dipole, and moderate basic interactions are possible. The greater the cyanopropyl content of the phase, the greater these interactions.
- SP®-2330, SLB®-IL76, SLB®-IL76i, SP®-2331, SP®-2380, SP®-2560, SP®-2340, SLB®-IL82, TCEP, SLB®-IL100
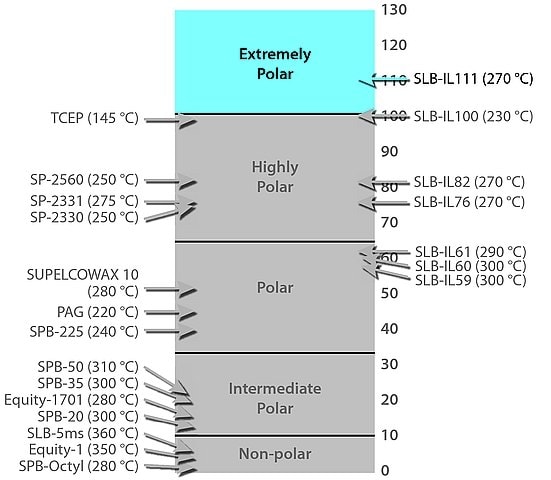
Extremely Polar
Extremely polar GC columns are made with the most selective of the GC stationary phases. They are commonly used to provide alternative selectivity of polarizable compounds. Another use is in GCxGC applications due to their orthogonal selectivity to non-polar columns. Elution order is determined by differences in the overall effects of possible interactions.
- Strong dispersive (van der Waals forces), very strong dipole-dipole, very strong dipole-induced dipole, and moderate basic interactions are possible.
- SLB®-IL111i, SLB®-ILD3606
To continue reading please sign in or create an account.
Don't Have An Account?