Environmental Monitoring and Aseptic Process Simulation
Patient safety is crucial, thus pharmaceutical products are manufactured under stringent controls. Microbial monitoring plays a vital role in GMP regulatory compliance, demonstrating that manufacturing processes, particularly in aseptic production, are well-managed.
Explore our comprehensive range of GMP-compliant solutions for microbial monitoring in aseptic pharmaceutical manufacturing, which includes monitoring of surfaces, personnel, and air. We also offer tailored solutions for isolators and RABS to enhance your workflow.
Additionally, we provide both granulated and ready-to-use culture media for media fills and aseptic process simulations, supporting classical environmental monitoring.
Section Overview
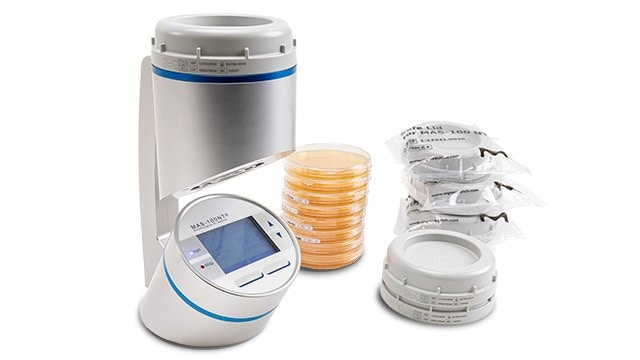
MICROBIAL AIR SAMPLERS
Our air samplers are based on different principles, enabling technology selection to suit your needs. We offer products specifically designed for explosion hazard areas, cleanrooms and isolators, and compressed gas monitoring. Our easy-to-use monitoring systems are designed for many environmental monitoring needs.
- The MAS-100 NT® and MAS-100 NT Ex® portable air samplers with or without HEPA exhaust filter provide excellent accuracy, precision and sampling efficiency. They are designed for highly critical areas, such as cleanrooms grade A
- The MAS-100 VF® active air sampler is designed for controlled environments. It is compact, easy to handle, and perfect for monitoring environment quality.
- Easily and automatically detect microbial contamination in gases with the MAS-100 Atmos® compressed gas sampler, which is simple, safe, and compliant with 21 CFR Part 11.
- The RCS® high-flow touch handheld air sampler features a light weight solution for ambient air monitoring. Impaction of microorganisms on centrigugal force avoids the need for Feller correction.The RCS® compressed gas adapter adds the capability for microbial monitoring in compressed gases, enabling seamless switching between viable air and compressed gas testing.
CONTACT PLATES, SETTLE PLATES, SLIDES AND SWABS
We provide a wide selection of plates tailored for environmental monitoring in isolators and critical cleanrooms, including the non-lockable ICR and lockable ICRplus Contact and Settle Plates with TSA + LTHTh.
- ICR Swabs are designed for absence-presence testing on dry surfaces in isolators and cleanrooms that are challenging to access for surface and personnel monitoring.
- ICR Contact plates are designed to minimize the risk of contamination as they are produced in clean rooms. The lockable ICRplus/RTplus versions can be safely transported for incubation under aerobic, microaerophilic, or anaerobic conditions.
- Hycon® Contact Slides are intended for monitoring flat and curved surfaces and personnel. They are available in standard single packaging or a double-bagged, gamma-irradiated format.
- ICR and ICRplus settle plates feature a high filling volume of 30 mL in 90 mm plates to reduce water loss during air monitoring. They are offered in animal-free formulations to minimize the risk of BSE/TSE contamination.
ISOLATORS AND RABS SOLUTIONS
Isolators and RABS are designed to eliminate human contact during controlled manufacturing. The MAS-100 Iso NT® & MAS-100 Iso MH® instruments, along with the IsoBag® rapid transfer bag, enable safe remote microbial air sampling and rapid culture media transfer into isolators.
The IsoBag® expedites environmental monitoring in aseptic production isolators by providing ready-to-use gamma-irradiated contact or settle plates for immediate use. This eliminates the need for decontamination cycles before introducing settle or contact plates, freeing up time for production processes and resulting in cost savings.
CULTURE MEDIA FOR ASEPTIC PROCESS SIMULATION
Our irradiated, triple-wrapped granulated media or ready-to-use media alleviate concerns about compromising validated processes during media fill trials.
- Granulated culture media, carefully formulated, enables pharmaceutical manufacturers to conduct efficient media fill trials with minimal risks.
- Ready-to-use culture media, such as Tryptic Soy Broth (TSB) and Vegetable Peptone Broth (VPB), undergo sterilization by autoclave and a two-step pre-filtration process, ensuring their quality.
Related Resources
- Brochure: Environmental Monitoring Solutions for Pharmaceutical Industry
GMP compliant solutions for process monitoring in pharmaceutical manufacturing
- Brochure: MAS-100® Air Samplers
With its various operation modes, the MAS-100 Atmos® instrument can adapt to different workflows. These modes include a freely accessible configuration, software-supported individual user management acc. to 21 CFR Part 11, and wireless options for embedding sample data into fully computerized workflows and software programs for compliant environmental monitoring.
- Data Sheet: Our Services Portfolio Supporting the MAS-100® Family
Discover our services portfolio supporting the MAS-100® family for active air monitoring
- Data Sheet: ICR/ICRplus Contact and Settle Plates
Designed to meet the highest requirements of microbial monitoring in sterile production environments
- Data Sheet: Media Fill Trials in Pharma
Granulated and Ready-to-Use Culture Media for Secure Aseptic Process Simulation (APS)
To continue reading please sign in or create an account.
Don't Have An Account?